Abstract
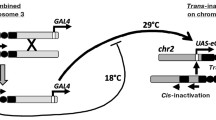
Autonomous P elements, inserted in heterochromatic telomeric associated sequences (TAS) at the X chromosome telomere (site 1A) have strong P element regulatory properties that include repression of P-induced hybrid-dysgenesis and of P-lacZ expression in the germline. P-lacZ insertions or defective P elements at 1A in TAS can also repress in trans a euchromatic P-lacZ in the germline. This property has been called a trans-silencing effect (TSE). It requires some sequence-homology between the telomeric insertion and the euchromatic transgene. When repression is partial, variegating lacZ expression is observed, suggesting a chromatin-based component. TSE is observed only when the silencer transgenes are maternally inherited and occurs only in the female germline. We have evidence that this silencing also works in the presence of homologous non-P element sequences suggesting that homology-dependent silencing could be a general phenomenon in the female germline; such a system might have been subsequently adopted by the P element family, allowing its own repression.
Similar content being viewed by others
References
Ajioka, J.W. & W.F. Eanes, 1989. The accumulation of P elements on the tip of the X chromosome in populations of Drosophila melanogaster. Gen. Res. 53: 1-6.
Anxolabéhère, D., H. Benes, D. Nouaud & G. Periquet, 1987. Evolutionary steps and transposable elements in Drosophila melanogaster: the missing RP strain obtained by genetic transformation. Evolution 41: 846-853.
Aravin, A.A., N.M. Naumova, A.V. Tulin, V.V. Vagin, Y.M. Rozovsky & V.A. Gvozdev, 2001. Double-stranded RNAmediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11: 1017-1027.
Biémont, C., F. Lemeunier, M.P. Garcia Guerreiro, J.F. Brookfield, G. Gautier, S. Aulard & E.G. Pasyukova, 1994. Populations dynamics of the copia, mdg1, mdg3, gypsy and P transposable elements in a natural population of Drosophila melanogaster. Genet. Res. 63: 197-212.
Blackman, R.K. & W.M. Gelbart, 1989. The transposable element hobo of Drosophila melanogaster, pp. 523-530 in Mobile DNA, edited by D.E. Berg & M.M. Howe. American Society for Microbiology, Washington, DC.
Boivin, A. & J.M. Dura, 1998. In vivo chromatin accessibility correlates with gene silencing in Drosophila. Genetics 150: 1539-1549.
Boivin, A., D. Anxolabéhère & S. Ronsseray, 2001. Polycomb and trithorax-group genes are modifiers of telomeric position effect. Poster, 5th 'Drosophila Heterochromatin Conference', June, Cortona, Italy.
Bregliano, J.C. & M.G. Kidwell, 1983. Hybrid Dysgenesis Determinants, edited by Shapiro. Academic Press, New York.
Cryderman, D.E., E.J. Morris, H. Biessmann, S.C.R. Elgin & L.L. Wallrath, 1999. Silencing at Drosophila telomeres: nuclear organization and chromatin structure play critical roles. EMBO J. 18: 3724-3735.
Csink, A.K. & S. Henikoff, 1996. Genetic modification of heterochromatic association and nuclear organization in Drosophila. Nature 381: 529-531.
Daniels, S.B., S.H. Clark, M.G. Kidwell & A. Chovnick, 1987. Genetic transformation of Drosophila melanogaster with an autonomous P element: phenotypic and molecular analyses of long established transformed lines. Genetics 115: 711-723.
Dernburg, A.F., K.W. Broman, J.C. Fung, W.F. Marshall, J. Philips, D.A. Agard & J.W. Sedat, 1996. Perturbation of nuclear architecture by long-distance chromosome interactions. Cell 77: 993-1002.
Dorer, D.R. & S. Henikoff, 1994. Expansion of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77: 993-1002.
Dorer, D.R. & S. Henikoff, 1997. Transgene repeats arrays interact with distant heterochromatin and cause silencing in cis and trans. Genetics 147: 1181-1190.
Engels, W.R., 1979. Hybrid dysgenesis in Drosophila melanogaster: rules of inheritance of female sterility. Genet. Res. 33: 219-236.
Engels, W.R., 1989. P elements in Drosophila, pp. 437-484 in Mobile DNA, edited by D.E. Berg & M.M. Howe. American Society for Microbiology, Washington, DC.
Fanti, L., D. Dorer, M. Berloco, S. Henikoff & S. Pimpinelli, 1998. Heterochromatin Protein 1 binds transgene arrays. Chromosoma 107: 286-292.
Finnegan, D.J., 1989. The I factor and I-R hybrid dysgenesis in Drosophila melanogaster, pp. 503-518 in Mobile DNA, edited by D.E. Berg & M.M. Howe. American Society for Microbiology, Washington, DC.
Gauthier, E., C. Tatout & H. Pinon, 2000. Artificial and epigenetic regulation of the I factor, a non-viral retrotransposon of Drosophila melanogaster. Genetics 156: 1867-1878.
Gloor, G.B., C.R. Preston, D.M. Jonhson-Schlitz, N.A. Nassif, R.W. Phillis, W.K. Benz, H.M. Robertson & W.R. Engels, 1993. Type I repressors of P element mobility. Genetics 135: 81-95.
Golubovsky, M.D., A.Y. Konev, M.F. Walter, H. Biessmann & J.M. Mason, 2001. Terminal retrotransposons activate a subtelomeric white transgene at the 2L telomere in Drosophila. Genetics 158: 1111-1123.
Jensen, S., M.P. Gassama & T. Heidmann, 1999a. Taming of transposable element by homology-dependent gene silencing. Nat. Genet. 21: 209-212.
Jensen, S., M.P. Gassama & T. Heidmann, 1999b. Cosuppression of I transposon activity in Drosophila by I-containing sense and antisense transgenes. Genetics 153: 1767-1774.
Karess, R.E. & G.M. Rubin, 1984. Analysis of P transposable element functions in Drosophila. Cell 38: 135-146.
Karpen, G.H. & A.C. Spradling, 1992. Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics 132: 737-753.
Kidwell, M.G., J.F. Kidwell & J.A. Sved, 1977. Hybrid dysgenesis in Drosophila melanogaster: a syndrome of aberrant traits including mutation, sterility, and male recombination. Genetics 86: 813-833.
Kobayashi, S., T. Kitamura, H. Sasaki & M. Okada, 1993. Two types of pole cells are present in the Drosophila embryo, one with and one without splicing activity for the third P element intron. Development 117: 885-893.
Lemaitre, B., S. Ronsseray & D. Coen, 1993. P cytotype repression of the P promoter is exclusively maternal in the germline: a model for P cytotype. Genetics 135: 149-160.
Levis, R., T. Hazelrigg & G.M. Rubin, 1985. Effects of genome position on the expression of transduced copies of the white gene of Drosophila. Science 229: 558-561.
Malinsky, S., A. Bucheton & I. Busseau, 2000. New insights on homology-dependent silencing of I factor activity by transgenes containing ORF1 in Drosophila melanogaster. Genetics 156: 1147-1155.
Marin, L.,M. Lehmann, D. Nouaud, H. Izaabel, D. Anxolabéhère & S. Ronsseray, 2000. P-element repression in Drosophila melanogaster by a naturally occurring defective telomeric P copy. Genetics 155: 1841-1854.
Matzke, M.A., A.J.M. Matzke & J.M. Kooter, 2001. RNA: guiding gene silencing. Science 293: 1080-1083.
Matzke, A.J.M., F. Neuhuber, Y.D. Park, P.F. Ambros & M.A. Matzke, 1994. Homology-dependent gene silencing in transgenic plants: epistatic silencing loci contain multiple copies of methylated transgenes. Mol. Gen. Genet. 244: 219-229.
Misra, S. & D.C. Rio, 1990. Cytotype control of Drosophila P element transcription: the 66 kD is a repressor of transposase activity. Cell 62: 269-284.
Misra, S., R.M. Buratowski, T. Ohkawa & D.C. Rio, 1993. Cytotype control of Drosophila melanogaster P element transposition: genomic position determines maternal repression. Genetics 135: 785-800.
Morel, J.B., P. Mourrain, C. Beclain & H. Vaucheret, 2000. DNA methylation and chromatin structure affect transcriptional and post-transcriptional transgene silencing in Arabidopsis. Curr. Biol. 10: 1591.
O'Hare, K. & G.M. Rubin, 1983. Structure of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell 34: 25-35.
Pal-Bhadra, M., U. Bhadra & J. Birchler, 1997. Cosuppression in Drosophila: gene silencing of Alcohol dehydrogenase by white-Adh transgenes is Polycomb dependent. Cell 90: 479-490.
Pal-Bhadra, M., U. Bhadra & J. Birchler, 1999. Cosuppression of nonhomologous transgene in Drosophila involves mutually related endogenous sequences. Cell 99: 35-46.
Preston, C.R. & W.R. Engels, 1989. Spread of P transposable element in inbred lines of Drosophila melanogaster. Prog. Nucl. Acid. Res. Mol. Biol. 36: 71-85.
Rio, D.C., F.A. Laski & G.M. Rubin, 1986. Identification and immunochemical analysis of biologically active Drosophila P element transposase. Cell 44: 21-32.
Robertson, H.M. & W.R. Engels, 1989. Modified P elements that mimic the P cytotype in Drosophila melanogaster. Genetics 123: 815-824.
Roche, S.E. & D.C. Rio, 1998. Trans-silencing by P elements inserted in subtelomeric heterochromatin involves the Polycomb-group gene, Enhancer of zeste. Genetics 149: 1839-1855.
Roche, S.E., M. Schiff & D.C. Rio, 1995. P-element repressor autoregulation involves germ-line transcriptional repression and reduction of third intron splicing. Genes Dev. 9: 1278-1288.
Ronsseray, S., M. Lehmann & D. Anxolabéhère, 1989. Copy number and distribution of P and I mobile elements in Drosophila melanogaster populations. Chromosoma 98: 207-214.
Ronsseray, S., M. Lehmann & D. Anxolabéhère, 1991. The maternally inherited regulation of P elements in Drosophila melanogaster can be elicited by two P copies at cytological site 1A on the X chromosome. Genetics 129: 501-512.
Ronsseray, S., A. Boivin & D. Anxolabéhère, 2001. P element repression in Drosophila melanogaster by variegating clusters of P-lac-white transgenes. Genetics 159: 1631-1642.
Ronsseray, S., M. Lehmann, D. Nouaud & D. Anxolabéhère, 1996. The regulatory properties of autonomous subtelomeric P elements are sensitive to a Suppressor of variegation in Drosophila melanogaster. Genetics 143: 1663-1674.
Ronsseray, S., M. Lehmann, D. Nouaud & D. Anxolabéhère, 1997. P element regulation and X-chromosome subtelomeric heterochromatin in Drosophila melanogaster. Genetica 100: 95-107.
Ronsseray, S., L. Marin, M. Lehmann & D. Anxolabéhère, 1998. Repression of hybrid dysgenesis in Drosophila melanogaster by combinations of telomeric P element reporters and naturally occurring P elements. Genetics 149: 1857-1866.
Selker, E.U., 1997. Epigenetic phenomena in filamentous fungi: useful paradigms or repeat-induced confusion? Trends Genet. 13: 293-295.
Simmons, M.J., J.D. Raymond, C.D. Grimes, C. Belinco, B.C. Haake, M. Jordan, C. Lund, T. Ojala & D. Papermaster, 1996. Repression of hybrid dysgenesis in Drosophila melanogaster by heat-shock-inducible sense and anti-sense P element constructs. Genetics 144: 1529-1544.
Slatis, H.M., 1955. A reconsideration of the brown-dominant position effect. Genetics 40: 246-251.
Thierry, D. & H. Vaucheret, 1996. Sequence homology requirement for transcriptional silencing of 35S transgenes and posttranscriptional silencing of nitrite reductase (trans)genes by the tobacco 271 locus. Plant Mol. Biol. 32: 1075-1083.
Vaucheret, H., 1993. Identification of a general silencer for 19S and 35S promoters in a transgenic tobacco plant: 90 bp of homology in the promoter sequence are sufficient for a trans-inactivation. C.R. Acad. Sci. Paris 316: 1471-1483.
Vaucheret, H., 1994. Promoter dependant trans-inactivation in transgenic tobacco plant: kinetic aspects of gene silencing and gene reactivation. C.R. Acad. Sci. Paris 317: 310-323.
Vaucheret, H. & M. Fagard, 2001. Transcriptional gene silencing in plants: targets inducers and regulators. Trends Genet. 17: 29-35.
Vaucheret, H., T. Elmayan, Ph. Mourrain & J.C. Palauqui, 1996. Analysis of tobacco transgene locus that triggers both transcriptional and posttransciptional silencing. Epigenetic Mechanisms of Gene Regulation. Cold Spring Harbor Laboratory Press, pp. 403-414.
Wallrath, L.L. & S.C.R. Elgin, 1995. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 9: 1263-1277.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ronsseray, S., Josse, T., Boivin, A. et al. Telomeric Transgenes and trans-Silencing in Drosophila . Genetica 117, 327–335 (2003). https://doi.org/10.1023/A:1022929121828
Issue Date:
DOI: https://doi.org/10.1023/A:1022929121828