Abstract
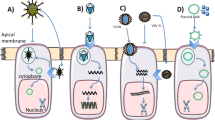
Cystic fibrosis is a disease for which a number of Phase I clinical trials of gene therapy have been initiated. Several factors account for the high level of interest in a gene therapy approach to this disease. CF is the most common lethal inherited disease in Caucasian populations. The lung, the organ that is predominantly responsible for the morbidity and mortality in CF patients, is accessible by a non-invasive method, the inhalation of aerosols. The vectors employed in the Phase I trials have included recombinant adenoviruses, adeno-associated viruses and cationic lipids. While there have been some positive results, the success of the vectors until now has been limited by either immunogenicity or low efficiency. A more fundamental obstacle has been the absence of appropriate receptors on the apical surface of airway epithelial cells. Molecular conjugates with carbohydrate substitution to provide targeting offer several potential advantages. Lactosylated polylysine in which 40% of the lysines have been substituted with lactose has been shown to provide a high efficiency of transfection in primary cultures of CF airway epithelial cells. Other important features include a relatively low immunogenicity and cytotoxicity. Most importantly, the lactosylated polylysine was demonstrated to give nuclear localization in CF airway epithelial cells. Until now, most non-viral vectors did not have the capability to provide nuclear localization. These unique qualities provided by the lactosylation of non-viral vectors, such as polylysine may help to advance the development of molecular conjugates sufficiently to warrant their use in future clinical trials for the gene therapy of inherited diseases of the lung.
Similar content being viewed by others
References
Welsh MJ, Tsui LC, Boat TF, Beaudet AL, Cystic fibrosis. In The Metabolic and Molecular Basis of Inherited Diseases, edited by Valle D (McGraw-Hill, New York, 1995), pp. 3799–876.
Robinson C, Scanlin TF, Cystic fibrosis. In Pulmonary Diseases and Disorders, edited by Fishman AP (McGraw-Hill, New York, 1997), pp. 1273–94.
Davies JC, Geddes DM, Alton EWFW, Gene therapy for cystic fibrosis, J Gene Med 3, 409–17 (2001).
Koehler DR, Hitt MM, Hu J, Challenges and strategies for cystic fibrosis lung gene therapy, Mol Ther 4, 84–91 (2001).
Huang L, Viroonchatapan E, Introduction. In Non-Viral Vectors for Gene Therapy, edited by Wagner E (Academic Press, San Diego, 1999), pp. 13–4.
Wivel NA, Wilson JM, Methods of gene therapy, Hematol Oncol Clin North Am 12, 483–99 (1998).
Wagner E, Zenke M, Cotton M, Beug H, Birnstiel ML, Transferinpolycation conjugates as carriers for DNA uptake into cells, Proc Natl Acad Sci USA 87, 3410–4 (1990).
Goldberg JC, Allen TD, Structural and functional organization of the nuclear envelope, Curr Opin Cell Biol 7, 301–9 (1995).
Talcott B, Moore MS, Getting across the nuclear pore complex, Trends Cell Biol 9, 312–8 (1999).
Wente SR, Gatekeepers of the nucleus, Science 288, 1374–6 (2000).
Wang G, Slepushkin V, Zabner J, Keshavjee S, Johnston JC, Sauter SL, Jolly DJ, Dubensky TJ Jr, Davidson BL, McCray PB Jr, Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect, J Clin Invest 104, R55–62 (1999).
Zuckerman JB, Robinson CB, McCoy KS, Shell R, Sferra TJ, Chirmule N, Magosin SA, Propert KJ, Brown-Parr EC, Hughes JV, Tazelaar J, Baker C, Goldman MJ, Wilson JM, A phase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis, Hum Gene Ther 10, 2973–85 (1999).
Wagner J, Reynolds T, Moran ML, Moss RB, Wine JJ, Flotte TR, Gardner P, Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus, Lancet 351, 1702–3 (1998).
Alton EWFW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, Davies JC, Smith SN, Browning J, Davies MG, Hodson ME, Durham SR, Li D, Jeffery PK, Scallan M, Balfour R, Eastman SJ, Cheng SH, Smith AE, Meeker D, Geddes DM, Cationic lipidmediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: A double-blind placebo-controlled trial, Lancet 353, 947–54 (1999).
Kollen WJW, Midoux P, Erbacher P, Yip A, Roche AC, Monsigny M, Glick MC, Scanlin TF, Gluconoylated and glycosylated polylysine as vectors for gene transfer into cystic fibrosis airway epithelial cells, Hum Gene Ther 7, 1577–86 (1996).
Kollen WJW, Mulberg AE, Wei X, Sugita M, Raghuram V, Wang J, Foskett JK, Glick MC, Scanlin TF, High-efficiency transfer of cystic fibrosis transmembrane conductance regulator cDNA into cystic fibrosis airway cells in culture using lactosylated polylysine as a vector, Hum Gene Ther 10, 615–22 (1999).
Gautam A, Densmore CL, Golunski E, Xu B, Waldrep JC, Transgene expression in mouse airway epithelium by aerosol gene therapy with PEI-DNA complexes, Mol Ther 3, 551–6 (2001).
Harvey BG, Maroni J, O'Donoghue KA, Chu KW, Muscat JC, Pippo AL, Wright CE, Hollman C, Wisnivesky JP, Kessler PD, Rasmussen HS, Rosengart TK, Crystal RG, Safety of local delivery of low-and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions, Hum Gene Ther 13, 15–6 (2002).
Crystal RG, McElvaney NG, Rosenfeld MA, Chu CS, Mastrangeli A, Hay JG, Brody SL, Jaffe HA, Eissa NT, Danel C, Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis, Nat Genet 8, 42–51 (1994).
Boucher RC, Status of gene therapy for cystic fibrosis lung disease, J Clin Invest 103, 441–5 (1999).
Pickles RJ, McCarty D, Matsui H, Hart PJ, Randell SH, Boucher RC, Limited entry of adenoviruses vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer, J Virol 72, 6014–23 (1998).
Sumerford C, Samulski RJ, Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions, J Vir 72, 1438–45 (1998).
Walters RW, Yi SMP, Keshavjee S, Brown KE, Welsh MJ, Chiorni JA, Zabner J, Binding of Adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer, J Biol Chem 276, 20610–6 (2001).
Kaludov N, Brown KE, Walters RW, Zabner J, Chiorni JA, Adeno-associated virus serotype 4 (AAV4) and Aav5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specifity, J Virol 75, 6884–93 (2001).
Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF, Endosomal processing limits gene transfer to polarized airway epithelia by adenoassociated virus, J Clin Invest 105, 1573–87 (2000).
Wang G, Sinn PL, McCray PBJ, Development of retroviral vectors for gene transfer to airway epithelia, Curr Opin Mol Ther 2, 497–506 (2000).
Crystal RG, Transfer of genes to humans: Early lessons and obstacles to success, Science 270, 404–10 (1995).
Li Z, Dullerman J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J, Forster M, Stocking C, Wahler A, Frank O, Ostertag W, Kuhlcke W, Eckert H-G, Fehse B, Baum C, Murine leukemia induced by retroviral gene marking, Science 296, 497 (2002).
Johnson LG, Retroviral approaches to gene therapy of cystic fibrosis, Ann NY Acad Sci 953, 43–52 (2001).
Kobinger GP, Weiner DJ, Yu QC, Wilson JM, Filovirus—pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo, Nat Biotechnol 19, 225–30 (2001).
Han S, Mahato RI, Sung YK, Kim SW, Development of biomaterials for gene therapy, Mol Ther 2, 302–17 (2000).
Yoshimura K, Rosenfeld MA, Nakamura H, Scherer EM, Pavirani A, Lecocq JP, Crystal JP, Expression of the human cystic fibrosis transmembrane conductance regulator gene in the mouse lung after in vivo intratracheal plasmid-mediated gene transfer, Nucl Acid Res 20, 3233–40 (1992).
Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, Durham SR, Jeffery PK, Hodson ME, Coutelle C, Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis, Nat Med 1, 39–46 (1995).
Porteous DJ, Dorin JR, McLachlan G, Davidson-Smith H, Davidson H, Stevenson BJ, Carothers AD, Wallace WA, Moralee S, Hoenes C, Kallmeyer G, Michaelis U, Naujoks K, Ho LP, Samways JM, Imrie M, Greening AP, Innes JA, Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis, Gene Ther 4, 210–8 (1997).
Noone PG, Hohneker KW, Zhou Z, Johnson LG, Foy C, Gipson C, Jones K, Noah TL, Leigh MW, Schwartzbach C, Efthimiou J, Pearlman R, Boucher RC, Knowles MR, Safety and biological efficacy of a lipid-CFTR complex for gene transfer in the nasal epithelium of adult patients with cystic fibrosis, Mol Ther 1, 105–14 (2000).
Hyde SC, Southern KW, Gileadi U, Fitzjohn EM, Mofford KA, Waddell BE, Gooi HC, Goddard CA, Hannavy K, Smyth SE, Egan JJ, Sorgi FL, Huang L, Cuthbert AW, Evans MJ, Colledge WH, Higgins CF, Webb AK, Gill DR, Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis, Gene Ther 7, 1156–65 (2000).
Flotte TR, Laube BL, Gene therapy in cystic fibrosis, Chest 120, 124S–31S (2001).
West J, Rodman DM, Gene therapy for pulmonary diseases, Chest 119, 613–7 (2001).
Brown MD, Schatzlein AG, Uchegbu IF, Gene delivery with synthetic (non viral) carriers, Int J Pharm 229, 1–21 (2001).
Cristiano RJ, Roth JA, Molecular conjugates: A targeted gene delivery vector for molecular medicine, J Mol Med 73, 479–86 (1995).
Sharon N, Lectins (Chapman and Hall, New York, 1989).
Sharon N, Lectin-carbohydrate complexes of plants and animals: An atomic view, Trends Biochem Sci 18, 221–6 (1993).
Weis WI, Drickamer K, Structural basis of lectin-carbohydrate recognition, Annu Rev Biochem 60, 443–73 (1996).
Ashwell G, Harford J, Carbohydrate-specific receptors of the liver, Ann Rev Biochem 51, 531–54 (1982).
Wu GY, Wu CH, Receptor-mediated in vitro gene transformation by a soluble DNA carrier system, J Biol Chem 262, 4429–32 (1987).
Wu GY, Wu CH, Receptor-mediated gene delivery and expression in vivo, J Biol Chem 263, 14621–4 (1988).
Perales JC, Ferkol T, Beegen H, Ratnoff OD, Hanson RW, Gene transfer in vivo: Sustained expression and regulation of genes introduced into the liver by receptor-taregeted uptake, Proc Natl Acad Sci USA 91, 4086–90 (1994).
Perales JC, Grossman GA, Molas M, Liu G, Ferkol T, Herpst J, Oda H, Hanson RW, Biochemical and functional characterization of DNA complexes capable of targetting gene to hepatocytes via the asialoglycoprotein receptor, J Biol Chem 272, 7398–407 (1997).
Zanta M, Boussif O, Abid A, Behr JP, In vitro gene delivery to hepatocytes with galactosyalted polyethylenimine, Bioconjug Chem 8, 839–44 (1997).
Bettinger T, Remy JS, Erbacher P, Size reduction of galactosylated PEI/DNA complexes improves lectin-mediated gene transfer into hepatocytes, Bioconjug Chem 10, 558–61 (1999).
Nishikawa M, Yamauchi M, Morimoto K, Ishida Y, Takakura Y, Hashida M, Hepatocyte-targeted in vivo gene expression by intravenous injection of plasmid DNA complexed with synthetic multi-functional gene delivery system, Gene Ther 7, 548–55 (2000).
Kawakami S, Fumoto S, Nishikawa M, Yamashita F, Hashida, In vivo gene delivery to the liver using novel galactosylated cationic liposomes, Pharm Res 17, 306–13 (2000).
Wileman T, Boshans R, Stahl P, Studies on ATP-dependent receptor-ligand dissociation, J Biol Chem 260, 7387–93 (1985).
Shepherd VL, Lee YC, Schlesinger PH, Stahl PD, L-Fucoseterminated glycoconjugates are recognized by pinocytosis receptors on macrophages, Proc Natl Acad Sci USA 78, 1019–22 (1981).
Erbacher P, Bousser MT, Raimond J, Monsigny M, Midoux P, Roche AC, Gene transfer by DNA/glycosylated polylysine complexes into human blood monocyte-derived macrophages, Hum Gene Ther 7, 721–9 (1996).
Ferkol T, Perales JC, Mularo F, Hanson RW, Receptor-mediated gene transfer into macrophages, Proc Natl Acad Sci USA 93, 101–5 (1996).
Nishikawa M, Takemura S, Yamashita F, Takakura Y, Meijer DK, Hashida M, Swart PJ, Pharmacokinetics and in vivo gene transfer of plasmid DNA complexed with mannosylated poly(L-lysine) in mice, J Drug Target 8, 29–38 (2000).
Ohashi T, Gene therapy for Gaucher disease, Nippon Rinsho 53, 3089–94 (1995).
Kohn DB, Sarver N, Gene therapy for HIV-1 infection, Adv Exp Med Biol 394, 421–8 (1996).
Erbacher P, Roche AC, Monsigny M, Midoux P, Putative role of chloroquine in gene transfer into a human hepatoma cell line by DNA/lactosylated polylysine complexes, Exp Cell Res 225, 186–94 (1996).
Kawakami S, Wong J, Sato A, Hattori Y, Yamashita F, Hashida M, Biodistribution characteristics of mannosylated, fucosylated, and galactosylated liposomes in mice, Biochim Biophys Acta 1524, 258–65 (2000).
Kawakami S, Wong J, Sato A, Hattori Y, Yamashita F, Hashida M, Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes, Gene Ther 7, 292–9 (2000).
Sato A, Kawakami S, Yamada M, Yamashita F, Hashida M, Enhanced gene transfection in macrophages using mannosylated cationic liposome-polyethylenimine-plasmid DNA complexes, J Drug Target 9, 201–7 (2001).
Bijsterbosch MK, Van Berkel TJ, Uptake of lactosylated lowdensity lipoprotein by galactose-specific receptors in rat liver, Biochem J 270, 233–9 (1990).
Bandyopadhyay P, Ma X, Linehan-Stieers C, Kren BT, Steer C, Nucleotide exchange in genomic DNA of Rat hepatocytes using RNA/DNA oligonucleotides, J Biol Chem 274, 10163–72 (1999).
Allo JC, Midoux P, Merten M, Souil E, Lipecka J, Figarella C, Monsigny M, Briand P, Fajac I, Efficient gene transfer into human normal and cystic fibrosis tracheal gland serous cells with synthetic vectors, Am J Respir Cell Mol Biol 22, 166–75 (2000).
Kollen WJW, Schembri FM, Gerwig GJ, Vliegenthart JFG, Glick MC, Scanlin TF, Enhanced efficiency of lactosylated poly-Llysine-mediated gene transfer into cystic fibrosis airway epithelial cells, Am J Respir Cell Mol Biol 20, 1081–6 (1999).
Fiume L, Di Stefano G, Busi C, Mattioli A, Battista Gervasi G, Bertini M, Bartoli C, Catalani R, Caccia G, Farina C, Fissi A, Pieroni O, Giuseppetti R, D'Ugo E, Bruni R, Rapicetta M, Hepatotropic conjugate of adenine arabinoside monophosphate with lactosaminated poly-L-lysine, J Hepatol 26, 253–9 (1997).
Di Stefano G, Busi C, Mattioli A, Fiume L, Selective delivery to the liver of antiviral nucleoside analogs coupled to a high molecular mass lactosaminated poly-L-lysine and administered to miceby intramuscular route, Biochem Pharmacol 49, 1769–75 (1995).
Klink DT, Chao S, Glick MC, Scanlin TF, Nuclear translocation of lactosylated poly-L-lysine/cDNAcomplex in cystic fibrosis airway epithelial cells, Mol Ther 3, 831–41 (2001).
Meyer KEB, Uyechi LS, Szoka FC Jr, Intracellular trafficking of nucleic acids. In Gene Therapy for Diseases of the Lung, edited by Brigham KL (Marcel Dekker, New York, 1997), pp. 135–80.
Dabauvalle MC, Schulz B, Scheer U, Peters R, Inhibition of nuclear accumulation of karyophilic proteins in living cells by the microinjection of the lectin wheat germ agglutinin, Exp Cell Res 174, 291–6 (1998).
Wilson GL, Dean BS, Wang G, AD, Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences, J Biol Chem 274, 22025–32 (1996).
Wang JL, Laing JG, Anderson RL, Lectins in the cell nucleus, Glycobiol 1, 209–14 (1991).
Wang JL, Werner EA, Laing JG, Patterson RJ, Nuclear and cytoplasmic localization of a lectin-ribonucleoprotein complex, Biochem Soc Trans 20, 269–74 (1992).
Laing JG, Wang JL, Identification of carbohydrate binding protein 35 in heterogeneous nuclear ribonucleoprotein complex, Biochem 27, 5329–34 (1988).
Gaudin JC, Mehul B, Hughes RC, Nuclear localisation of wild type and mutant galectin-3 in transfected cells, Biol Cell 92, 49–58 (2000).
Dagher SF, Wang JL, Patterson RJ, Identification of galectin-3 as a factor in pre-mRNA splicing, Proc Natl Acad Sci USA 92, 1213–7 (1995).
Richardson WD, Mills AD, Dilworth DM, Laskey RA, Dingwall M, Nuclear protein migration involves two steps: Rapid binding at the nuclear envelope followed by slower translocation through nuclear pores, Cell 52, 655–64 (1988).
Menon RP, Strom M, Hughes RC, Interaction of a novel cysteine and histidine-rich cytoplasmic protein with galectin-3 in a carbohydrate-independent manner, FEBS Ltr 470, 227–31 (2000).
Greber UF, Suomalainen, Stidwill RP, Boucke K, Ebersold MW, Helenius A, The role of the nuclear core complex in adenovirus DNA entry, EMBO J 16, 5998–6007 (1997).
Wagner E, Cotton M, Foisner R, Beug H, Birnstiel ML, Transferrin-polycation-DNAcomplexes: The effect of polycations on the structure of the complex and DNA delivery to cells, Proc Natl Acad Sci USA 89, 7934–8 (1991).
de Lima MC, Gene delivery mediated by cationic liposomes: From biophysical aspects to enhancement of transfection, Mol Membr Biol 16, 103–9 (1999).
Chan KC, Jans DA, Enhancement of polylysine mediated transferrinfection by nuclear localization of sequences: Polylysine does not function as a nuclear localization sequence, Hum Gene Ther 10, 1695–702 (1999).
Rout MP, Aitchison JD, The nuclear pore complex as a transport machine, J Biol Chem 276, 16593–6 (2001).
Anderson WF, Human gene therapy, Nature 392, 25–30 (1998).
Knowles MR, Hohneker KW, Zhou ZQ, Olsen JC, Noah TL, Hu PC, Leigh MW, Engelhardt JF, Edwards LJ, Jones KR, Grossman M, Wilson JM, Johnson LG, Boucher RC, A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelial of patients with cystic fibrosis, N Engl J Med 384, 823–31 (1995).
Bellon G, Michel-Calemard L, Thouvenot D, Jagneaux V, Poitevin F, Malcus C, Accart N, Layani MP, Aymard M, Bernon H, Bienvenu J, Courtney M, Doring G, Gilly B, Lamy D, Levrey H, Morel Y, Paulin C, Perraud F, Rodillon L, Seme C, So S, Touraine-Moulin F, Schatz C, Pavirani A, Aerosol administration of a recombinant adenovirus expressing CFTR to ystic fibrosis patients: A phase I clinical trial, Hum Gene Ther 8, 15–25 (1997).
Harvey BG, Leopold LP, Hackett NR, Grasso TM, Williams PM, Tucker AL, Kaner RJ, Ferris B, Gonda I, Sweeney TD, Ramalingam R, Kovesdi I, Crystal RG, Airway epithelial CFTR mRNAexpression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus, J Clin Invest 104, 1245–55 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klink, D.T., Glick, M.C. & Scanlin, T.F. Gene therapy of cystic fibrosis (CF) airways: A review emphasizing targeting with lactose. Glycoconj J 18, 731–740 (2001). https://doi.org/10.1023/A:1020879524587
Issue Date:
DOI: https://doi.org/10.1023/A:1020879524587