Abstract
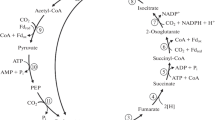
Major pathways of carbon metabolism were studied in strains D-402 and D-405 of freshwater colorless sulfur bacteria of the genus Beggiatoa grown organotrophically and mixotrophically. The bacteria were found to possess all the enzymes of the tricarboxylic acid (TCA) and glyoxylate cycles. When organotrophic growth changed to mixotrophic growth, the activity of the TCA cycle enzymes decreased 2- to 3-fold, but the activity of enzymes of the glyoxylate cycle increased threefold. It follows that, in the oxidation of thiosulfate, organic compounds no longer play the leading part in the energy metabolism, and most of electrons that enter the electron transport chain (ETC) derive from inorganic sulfur compounds. A connection was established between the structure and kinetic characteristics of malate dehydrogenase—an enzyme of the TCA and glyoxylate cycles—and the type of carbon metabolism in the strains studied. Malate dehydrogenase in organotrophically grown cells of strains D-402 and D-405 is dimeric, whereas in strain D-402 grown mixotrophically it is tetrameric.
Similar content being viewed by others
REFERENCES
Patritskaya, V.Yu., Regulation of the Carbon and Sulfur Metabolism in Filamentous Gliding Sulfur Bacteria of the Genera Beggiatoa and Leucothrix, Cand. Sci. (Biol.) Dissertation, Moscow, Inst. Microbiol., Russ. Acad. Sci., 2001.
Grabovich, M.Yu., Dubinina, G.A., Lebedeva, V.Yu., and Churikova, V.V., Mixotrophic and Lithoheterotrophic Growth of the Freshwater Filamentous Sulfur Bacterium Beggiatoa leptomitiformis D-402, Mikrobiologiya, 1998, vol. 57, no. 4, pp. 383–388.
Grabovich, M.Yu., Patritskaya, V.Yu., Muntyan, M.S., and Dubinina, G.A., Lithoautotrophic Growth of the Freshwater Strain Beggiatoa D-402 and Energy Conservation Culture under Microoxic Conditions, FEMS Microbiol. Lett., 2001, vol. 204, pp. 341–345.
Friedrich, P., Enzymes: Quaternary Structure and beyond: An Introduction to Supramolecular Enzymes Organization, Budapest: Akademiai Kiado, 1983.
Labrou, N.E. and Clonis, Y.D., L-Malate Dehydrogenase from Pseudomonas stutzeri: Purification and Characterization, Arch. Biochem. Biophys., 1997, vol. 337, pp.-103–114.
Rolstand, A.K., Howland, E., and Sirenag, R., Malate Dehydrogenase from the Thermophilic Green Bacterium Chloroflexus aurantiacus: Purification, Molecular Weight, Amino Acid Composition, and Partial Amino Acid Sequence, J. Bacteriol., 1988, vol. 170, pp. 2947–2953.
Tayeh, M.A. and Madigan, M.T., Malate Dehydrogenases in Phototrophic Purple Bacteria: Thermal Stability, Amino Acid Composition and Immunological Properties, Biochem. J., 1988, vol. 252, pp. 595–600.
Janiczek, O., Kovar, J., and Glatz, Z., Purification and Properties of Malate Dehydrogenase from Paracoccus denitrificans, Prep. Biochem., 1993, vol. 23, pp. 285–301.
Grabovich, M.Yu., Dubinina, G.A., Churikova, V.V., Churikov, S.N., Korovina, T.I., and Glushkov, A.F., A Study of the Carbon Metabolism of Beggiatoa leptomitiformis during Chemoorganoheterotrophic Growth, Mikrobiologiya, 1993, vol. 62, no. 3, pp. 430–435.
Grabovich, M.Yu., Dubinina, G.A., Churikova, V.V., Glushkov, A.F., and Churikov, S.N., Peculiarities of the Carbon Metabolism in the Colorless Sulfur Bacterium Macromonas bipunctata, Mikrobiologiya, 1993, vol. 62, no. 3, pp. 421–429.
Determan, G., Gel'-khromatografiya (Gel Chromatography), Moscow: Mir, 1970.
Nesterenko, M.V., Tilley, M., and Upton, S.J., A Simple Modification of Blum's Silver Stain Method Allows for 30 Minute Detection of Proteins in Polyacrylamide Gels, J. Biochem. Biol., 1994, vol. 28, pp. 239–242.
Zemlyanukhin, A.A. and Zemlyanukhin, L.A., Bol'shoi praktikum po fiziologii i biokhimii rastenii (A Practical Course of Plant Physiology and Biochemistry), Vornezh, Voronezh Gos. Univ., 1996.
Gaal, O., Medgyesi, G.A., and Vereczkey, L., Electrophoresis in the Separation of Biological Macromolecules, Chichester: Wiley, 1980.
Eprintsev, A.T., Igamberdiev, A.U., and Ashnin, L., The Malate Dehydrogenase System of Wolfia arrhiza: Characterization and Role in the Adaptation to Light and Darkness, Fiziol. Rast. (Moscow), 1996, vol. 43, pp. 36–42.
Chekanova, Yu.A., The Interrelation of Sulfur Compound Oxidation with the Operation of the Respiratory Chain in Macromonas bipunctata and Beggiatoa leptomitiformis Strain D-405, Cand. Sci. (Biol.) Dissertation, Moscow, Inst. Microbiol., Russ. Acad. Sci., 1991.
Hunter, G.R., Hellman, U., Cazzulo, J.J., and Nowicki, ?C., Tetrameric and Dimeric Malate Dehydrogenase Isoenzymes in Trypanosoma cruzi Epimastigotes, Mol. Biochem. Parasitol., 2000, vol. 105, pp. 203–214.
Pinheiro de Carvalho, M.A., Zemlyanukhin, A.A., and Eprintsev, A.T., Malatdegidrogenaza vysshikh rastenii (Malate Dehydrogenase of Higher Plants), Voronezh: Voronezh Gos. Univ., 1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stepanova, I.Y., Eprintsev, A.T., Falaleeva, M.I. et al. Dependence of Malate Dehydrogenase Structure on the Type of Metabolism in Freshwater Filamentous Colorless Sulfur Bacteria of the Genus Beggiatoa. Microbiology 71, 377–382 (2002). https://doi.org/10.1023/A:1019825006330
Issue Date:
DOI: https://doi.org/10.1023/A:1019825006330