Abstract
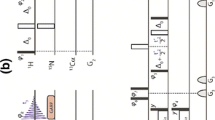
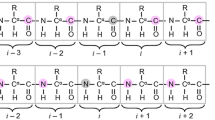
Resonance overlap in 13Cα-dimension can seriously deteriorate sequential assignment of proteins, especially in the case of highly alpha helical or partially unfolded structures. In this paper, two novel triple-resonance experiments, for obtaining solely intraresidual HN, N, Cα correlations, are introduced. The proposed experiments are complementary to the conventional HN(CO)CA experiment, and can be utilized for the sequential assignment of 15N/13C/(2H)-labeled proteins. Coherence transfer efficiency of the new experiment is comparable to the conventional HNCA experiment on proteins with sufficiently long 15N transverse relaxation time. These new coherence transfer schemes are also very useful building blocks for experiments gathering structural information, such as J-couplings, exclusively on the intraresidual alpha carbon. Experimental assessment is demonstrated on ubiquitin at 600 1H MHz.
Similar content being viewed by others
References
Asla Ltd. http://www.asla-biotech.com
Bax, A. and Grzesiek, S. (1993) Acc. Chem. Res., 26, 131–138.
Bax, A. and Ikura, M. (1991) J. Biomol. NMR, 1, 99–104.
Chiarparin, E., Pelupessy, P., Ghose, R. and Bodenhausen, G. (2000) J. Am. Chem. Soc., 122, 1758–1761.
Delaglio, F., Torchia, D.A. and Bax, A. (1991) J. Biomol. NMR, 1, 439–446.
Grzesiek, S. and Bax, A. (1992a) J. Magn. Reson. 99, 201–207.
Grzesiek, S. and Bax, A. (1992b) J. Am. Chem. Soc. 114, 6291–6293.
Grzesiek, S. and Bax, A. (1993a) J. Biomol. NMR 3, 185–204.
Grzesiek, S. and Bax, A. (1993b) J. Am. Chem. Soc., 115, 12593–12594.
Kay, L.E., Ikura, M., Tschudin, R. and Bax, A. (1990) J. Magn. Reson., 89, 496–514.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Logan, T.M., Olejniczak, E.T., Xu, R.X. and Fesik, S.W. (1993) J. Biomol. NMR, 3, 225–231.
Loria, J.P., Rance, M. and Palmer III, A.G. (1999) J. Magn. Reson., 141, 180–184.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989) J. Magn. Reson., 85, 393–399.
Meissner, A., Schulte-Herbrüggen, T., Briand, J. and Sørensen, O.W. (1998) Mol. Phys., 95, 1137–1142.
Panchal, S.C., Bhavesh, N.S. and Hosur, R.V. (2001) J. Biomol. NMR, 20, 135–147.
Permi, P., Sorsa, T., Kilpeläinen, I. and Annila, A. (1999) J. Magn. Reson., 141, 44–51.
Permi, P. and Annila, A. (2001a) Magn. Res. Chem., 39, 179–181.
Permi, P. and Annila, A. (2001b) J. Biomol. NMR, 20, 127–133.
Permi, P. and Annila, A. (2002) J. Magn. Reson., 155, 123–130.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1997) Proc. Natl. Acad. Sci. USA, 94, 12366–12371.
Pervushin, K.V., Wider, G. and Wüthrich, K. (1998) J. Biomol. NMR, 12, 345–348.
Piantini, U., Sørensen, O.W. and Ernst, R. (1982) J. Am. Chem. Soc., 104, 6800–6801.
Rance, M., Loria, P. and Palmer, A.G., III. (1999) J. Magn. Reson., 136, 92–101.
Sattler, M., Schleucher, J. and Griesinger, C. (1999) Prog. Nucl. Magn. Reson. Spectr., 34, 93–158.
Schleucher, J., Sattler, M. and Griesinger, C. (1993) Angew. Chem. Int. Ed. Engl., 32, 1489–1491.
Shaka, A.J., Keeler, J., Frenkiel, T. and Freeman, R. (1983) J. Magn. Reson., 52, 335–338.
Shaka, A.J., Parker, P.B. and Freeman, R. (1985) J. Magn. Reson., 64, 547–552.
Shan, X., Gardner, K.H., Muhandiram, D.R., Rao, N.S., Arrowsmith, C.H., and Kay, L.E. (1996) J. Am. Chem. Soc., 118, 6570–6579.
Weigelt, J. (1998) J. Am. Chem. Soc., 120, 10778–10779.
Wittekind, M. and Mueller, L. (1992) J. Magn. Reson., 101B, 201–205.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, John Wiley and Sons, New York, NY.
Yamazaki, T., Lee, W., Revington, M., Mattiello, D.L., Dahlquist, F.W., Arrowsmith, C.H. and Kay, L.E. (1994) J. Am. Chem. Soc., 116, 6464–6465.
Yamazaki, T., Tochio, H., Furui, J., Aimoto, S. and Kyogoku, Y. (1997) J. Am. Chem. Soc., 120, 872–880.
Yang, D. and Kay, L.E. (1999a) J. Am. Chem. Soc., 121, 2571–2575.
Yang, D. and Kay, L.E. (1999b) J. Biomol. NMR, 13, 3–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Permi, P. Intraresidual HNCA: An experiment for correlating only intraresidual backbone resonances. J Biomol NMR 23, 201–209 (2002). https://doi.org/10.1023/A:1019819514298
Issue Date:
DOI: https://doi.org/10.1023/A:1019819514298