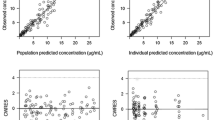
Abstract
Etoposide is used to treat childhood malignancies, and its plasma pharmacokinetics have been related to pharmacodynamic endpoints. Limiting the number of samples should facilitate the assessment of etoposide pharmacokinetics in children. We compared limited sampling strategies using multiple linear regression of plasma concentrations and clearance with Bayesian methods of estimating clearance using compartmental pharmacokinetic models. Optimal sampling times were estimated in the multiple linear regression method by determining the combination of two samples which maximized the correlation coefficient, and in the Bayesian estimation approach by minimizing the variance in estimates of clearance. Clearance estimates were compared to the actual clearances from Monte Carlo-simulated data and predicted clearances estimated using all available plasma concentrations in clinical data from children with acute lymphoblastic leukemia. Multiple linear regression poorly predicted clearance (mean bias 8.3%, precision 17.5%), but improved if plasma concentrations were logarithmically transformed (mean bias 1.4%, precision 12.5%). Bayesian estimation methods with optimal samples gave the best overall prediction (mean bias 2.5%, precision 6.8%) and also performed better than regression methods for abnormally high or low clearances. We conclude that Bayesian estimation with limited sampling gives the best estimates of etoposide clearance.
Similar content being viewed by others
REFERENCES
B. Desoize, F. Marechal, and A. Cattan. Clinical pharmacokinetics of etoposide during 120 hr continuous infusions in solid tumours. Br. J. Cancer 62:840-841 (1990).
M. V. Relling, H. McLeod, L. Bowman, and V. M. Santana, Etoposide pharmacokinetics and pharmacodynamics after acute and chronic exposure to cisplatin. Clin. Pharmacol. Ther. 56:503-511 (1994).
K. Kobayashi and M. J. Ratain. Pharmacodynamics and long-term toxicity of etoposide. Cancer Chemother. Pharmacol. 34 Suppl.:S64-8 (1994).
D. S. Sonnichsen, R. C. Ribeiro, X. Luo, P. Mathew, and M. Relling, Pharmacokinetics and pharmacodynamics of 21 day continuous oral etoposide in pediatric solid tumor patients. Clin. Pharmacol. Ther. 58:99-107 (1995).
H. Minami, M. J. Ratain, Y. Ando, and K. Shimokata. Pharmacodynamic modeling of prolonged administration of etoposide. Cancer Chemother. Pharmacol. 39:61-66 (1996).
D. Gentili, M. Zucchetti, V. Torri, C. Sessa, J. de Jong, F. Cavalli, and M. D'Incalci. A limited sampling model for the pharmacokinetics of etoposide given orally. Cancer Chemother. Pharmacol. 32:482-486 (1993).
B. Tranchand, C. Amsellem, E. Chatelut, G. Freyer, A. Iliadis, B. Ligneau, V. Trillet-Lenoir, P. Canal, I. Lochon, and C. J. Ardiet. A limited-sampling strategy for estimation of etoposide pharmacokinetics in cancer patients. Cancer Chemother. Pharmacol. 43:316-322 (1999).
J. B. Holz, H. Koppler, L. Schmidt, H. W. Fritsch, K. H. Pfluger, and H. Jungclas. Limited sampling models for reliable estimation of etoposide area under the curve. Eur. J Cancer 31A:1794-1798 (1995).
B. L. Lum, K. J. Lane, T. W. Synold, A. Goram, S. B. Charnick, and B. I. Sikic, Validation of a limited sampling model to determine etoposide area under the curve. Pharmacotherapy 17:887-890 (1997).
I. Mahmood and R. Miller. Comparison of the Bayesian approach and a limited sampling model for the estimation of AUC and Cmax: a computer simulation analysis. Int. J Clin. Pharmacol. Ther. 37:439-445 (1999).
E. Walter and L. Pronzato. Optimal experiment design for nonlinear models subject to large prior uncertainties. Am. J Physiol. 253:R530-R534 (1987).
D. Z. D'Argenio. Incorporating prior parameter uncertainty in the design of sampling schedules for pharmacokinetic parameter estimation experiments. Math. Biosci. 99:105-118 (1990).
E. Walter and L. Pronzato. Qualitative and quantitative experiment design for phenomenological models-A survey. Automatica 26:195-213 (1990).
G. D. Swanson. On the single-point, single-dose problem. In: Advanced Methods of Pharmacokinetic and Pharmacodynamic Systems Analysis. Plenum Press, New York, 129-135 (1991)
Y. Merle and F. Mentre. Bayesian design criteria: computation, comparison, and application to a pharmacokinetic and a pharmacodynamic model. J. Pharmacokinet. Biopharm. 23:101-125 (1995).
Y. Merle and F. Mentre. Stochastic optimization algorithms of a Bayesian design criterion for Bayesian parameter estimation of nonlinear regression models: application in pharmacokinetics. Math. Biosci. 144:45-70 (1997).
D. Z. D'Argenio. Optimal sampling times for pharmacokinetic experiments. J. Pharmacokinet. Biopharm. 9:739-756 (1981).
G. E. P. Box and H. L. Lucas, Design of experiments in nonlinear situations. Biometrika 46:77-90 (1959).
D. Z. D'Argenio and A. Schumitzky. ADAPT II User's Guide: Pharmacokinetic_Pharmacodynamic Systems Analysis Software (1997).
J. Boos, E. Real, P. Schulze-Westhof, J. Wolff, T. Euting, and H. Jürgens. Investigation of the variability of etoposide pharmacokinetics in children. Int. J. Clin. Pharmacol. Ther.-Toxicol. 30:495-497 (1992).
M. V. Relling, Y. Yanishevski, J. Nemec, W. E. Evans, J. M. Boyett, F. G. Behm, and C. H. Pui. Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia. Leukemia 12:346-352 (1998).
S. P. Lowis, A. D. J. Pearson, D. R. Newell, and M. Cole, Etoposide pharmacokinetics in children: The development and prospective validation of a dosing equation. Cancer Res. 53:4881-4889 (1993).
X. Cai, M. H. Woo, M. J. Edick, and M. V. Relling, Simultaneous quantitation of etoposide and its catechol metabolite in human plasma using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B. Biomed. Sci. Appl. 728:241-250 (1999).
L. B. Sheiner and S. L. Beal, Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 9:503-512 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Panetta, J.C., Wilkinson, M., Pui, CH. et al. Limited and Optimal Sampling Strategies for Etoposide and Etoposide Catechol in Children with Leukemia. J Pharmacokinet Pharmacodyn 29, 171–188 (2002). https://doi.org/10.1023/A:1019755608555
Issue Date:
DOI: https://doi.org/10.1023/A:1019755608555