Abstract
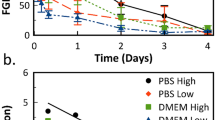
The design of an aqueous formulation for acidic fibroblast growth factor (aFGF) requires an understanding of the type of compounds that can either directly or indirectly stabilize the protein. To this end, spectrophotometric turbidity measurements were initially employed to screen the ability of polyanionic ligands, less specific compounds, and variations in solution conditions (temperature and pH) to stabilize aFGF against heat-induced aggregation. It was found that in addition to the well-known protection of aFGF by heparin, a surprisingly wide variety of polyanions (including small sulfated and phosphorylated compounds) also stabilizes aFGF. These polyanionic ligands are capable of raising the temperature at which the protein unfolds by 15–30°C. Many commonly used excipients were also observed to stabilize aFGF in both the presence and the absence of heparin. High concentrations of some of these less specific agents are also able to increase the temperature of aFGF thermal unfolding by as much as 6–12°C as shown by circular dichroism and differential scanning calorimetry. Other compounds were found which protect the chemically labile cysteine residues of aFGF from oxidation. Aqueous formulations of aFGF were thus designed to contain both a polyanionic ligand that enhances structural integrity by binding to the protein and chelating agents (e.g., EDTA) to prevent metal ion-catalyzed oxidation of cysteine residues. While room-temperature storage (30°C) leads to rapid inactivation of aFGF in physiological buffer alone, several of these aFGF formulations are stable in vitro for at least 3 months at 30°C. Three aFGF topical formulations were examined in an impaired diabetic mouse model and were found to be equally capable of accelerating wound healing.
Similar content being viewed by others
REFERENCES
W. H. Burgess and T. Maciag. The heparin-binding (fibroblast) growth factor family of proteins. Annu. Rev. Biochem. 58:575–606 (1989).
A. Baird and P. Böhlen. Fibroblast growth factors. In M. B. Sporn and A. B. Roberts (eds.), Peptide Growth Factors and Their Receptors 1, Springer-Verlag, Berlin, 1990, pp. 369–418.
P. ten Dijke and K. K. Iwata. Growth factors for wound healing. Biotechnology 7:793–798 (1989).
X. Zhu, H. Komiya, A. Chirino, S. Faham, G. M. Fox, T. Arakawa, B. T. Hsu, and D. C. Rees. Three-dimensional structures of acidic and basic fibroblast growth factors. Science 251:90–93 (1991).
A. E. Eriksson, L. S. Cousens, L. H. Weaver, and B. W. Matthews. Three-dimensional structure of human basic fibroblast growth factor. Proc. Natl. Acad. Sci. USA 88:3441–3445 (1991).
J. Zhang, L. S. Cousens, P. J. Barr, and S. R. Sprang. Three-dimensional structure of human fibroblast growth factor, a structural homolog of interleukin-1β. Proc. Natl. Acad. Sci. USA 88:3446–3450 (1991).
T. Ago, Y. Kitagawa, A. Fujishima, Y. Matsura, and Y. Katsube. Crystal structure of basic fibroblast growth factor at 1.6 Å resolution. J. Biochem. 110:360–363 (1991).
D. Gospodarowicz and J. Cheng. Heparin protects basic and acidic FGF from inactivation. J. Cell. Physiol. 128:475–484 (1986).
T. K. Rosengart, W. V. Johnson, R. Friesel, R. Clark, and T. Maciag. Heparin protects heparin-binding growth factor-1 from proteolytic inactivation in vitro. Biochem. Biophys. Res. Commun. 152:432–440 (1988).
O. Saksela, D. Moscatelli, A. Sommer, and D. B. Rifkin. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J. Cell Biol. 107:743–751 (1988).
S. N. Mueller, K. A. Thomas, J. DiSalvo, and E. M. Levine. Stabilization by heparin of acidic fibroblast growth factor mitogenicity for human endothelial cells in vitro. J. Cell. Physiol. 140:439–448 (1989).
A. Sommer and D. B. Rifkin. Interaction of heparin with human basic fibroblast growth factor: Protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J. Cell. Physiol. 138:215–220 (1989).
K. Vlodavsky, Z. Fuks, R. Ishai-Michaeli, P. Bashkin, E. Levi, G. Korner, R. Bar-Shavit, and M. Klagsbrun. Extracellular matrix-resident basic fibroblast growth factor: Implication for the control of angiogenesis. J. Cell Biochem. 45: 167–176 (1991).
Y. Nakanishi, K. Kihara, K. Mizuno, Y. Masamune, Y. Yoshitake, and K. Nishikawa. Direct effect of basic fibroblast growth factor on gene transcription in a cell-free system. Proc. Natl. Acad. Sci. USA 89:5216–5220 (1992).
R. Wetzel, L. J. Perry, M. G. Mulkerrin, and L. M. Randall. Unfolding and inactivation: Genetic and chemical approaches to the stabilization of T4 lysozyme and human interferon gamma against irreversible thermal denaturation. In J. B. Hook, G. Poste, and J. Schatz (eds.), Protein Design and the Development of New Therapeutics and Vaccines, Plenum Press, New York, 1990, pp. 79–115.
D. G. Greenhalgh, K. H. Strugel, M. J. Murray, and R. Ross. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am. J. Pathol. 163:1235–1246 (1990).
D. L. Linemeyer, J. G. Menke, L. J. Kelly, J. DiSalvo, D. Soderman, M. T. Schaeffer, S. Ortega, G. Gimenez-Gallego, and K. A. Thomas. Disulfide bonds are neither required, present, nor compatible with full activity of human recombinant acidic fibroblast growth factor. Growth Factors 3:287–298 (1990).
S. Ortega, M. T. Schaeffer, D. Soderman, J. DiSalvo, D. L. Linemeyer, G. Gimenez-Gallego, and K. A. Thomas. Conversion of cysteine to serine residues alters the activity, stability, and heparin dependence of acidic fibroblast growth factor. J. Biol. Chem. 266:5842–5846 (1991).
S. Yamazaki, F. Leu, A. Lee, E. Scattergood, K. Thompson, R. Middaugh, R. Sitrin, and M. King. A novel technique for controlled folding of recombinant aFGF using cross-flow dialysis. FASEB J 5:1186 (1991).
S. Yamazaki and P. DePhillips. Method for the purification of therapeutically active recombinant acidic fibroblast growth factor. Eur. Patent Appl. No. 90201870.4 (1990).
R. A. Copeland, H. Ji, A. J. Halfpenny, R. W. Williams, K. C. Thompson, W. K. Herber, K. A. Thomas, M. W. Bruner, J. A. Ryan, D. Marquis-Omer, G. Sanyal, R. D. Sitrin, S. Yamazaki, and C. R. Middaugh. The structure of human acidic fibroblast growth factor and its interaction with heparin. Arch. Biochem. Biophys. 289:53–61 (1991).
W. H. Burgess. Structure-function studies of acidic fibroblast growth factor. Ann. N.Y. Acad. Sci. 638:89–97 (1992).
B. Steadman, P. A. Trautman, E. Q. Lawson, M. J. Raymond, D. A. Mood, J. A. Thomson, and C. R. Middaugh. A differential scanning calorimetric study of the bovine lens crystallins. Biochemistry 28:9653–9658 (1989).
B. Matuszewska, M. Keogan, J. V. Bondi, D. Fisher, K. Soper, C. M. Hoe, and C. Huber. Acidic fibroblast growth factor: Evaluation of topical formulations in the diabetic mouse wound healing model (submitted for publication).
J. M. Dabora, G. Sanyal, and C. R. Middaugh. Effect of polyanions on the refolding of human acidic fibroblast growth factor. J. Biol. Chem. 266:23637–23640 (1991).
D. B. Volkin, P. K. Tsai, J. M. Dabora, and C. R. Middaugh. The effect of polyanions on the stabilization of acidic fibroblast growth factor. In M. R. Ladisch and A. Bose (eds.), Harnessing Biotechnology for the 21st Century, American Chemical Society, Washington, DC, 1992, pp. 290–293.
D. B. Volkin, P. K. Tsai, J. M. Dabora, J. O. Gress, C. J. Burke, R. J. Linhardt, and C. R. Middaugh. The physical stabilization of acidic fibroblast growth factor by polyanions. Arch. Biophys. Biochem. 300:30–41 (1993).
S. N. Timasheff and T. Arakawa. Stabilization of protein structure by solvents. In T. E. Creighton (ed.), Protein Structure, A Practical Approach, IRL Press, Oxford, 1989, pp 331–345.
J. C. Lee and S. N. Timasheff. The stabilization of proteins by sucrose. J. Biol. Chem. 256:7193–7201 (1981).
D. B. Volkin and A. M. Klibanov. Minimizing protein inactivation. In T. E. Creighton (ed.), Protein Function, A Practical Approach, IRL Press, Oxford, 1989, pp. 1–24.
J. Wu, J. T. Yang, and C. C. S. Wu. β-II conformation of all β-proteins can be distinguished from unordered form by circular dichroism. Anal. Biochem. 200:359–364 (1992).
C. R. Middaugh, H. Mach, C. J. Burke, D. B. Volkin, J. M. Dabora, P. K. Tsai, M. W. Bruner, J. A. Ryan, and K. E. Marfia. Nature of the interaction of growth factors with suramin. Biochemistry 31:9016–9024 (1992).
M. Seno, R. Sasada, M. Iwane, K. Sudo, T. Kurokawa, K. Ito, and K. Igarashi. Stabilizing basic fibroblast growth factor using protein engineering. Biochem. Biophys. Res. Comm. 151:701–708 (1988).
K. A. Engleka and T. Maciag. Inactivation of human fibroblast growth factor-1 (FGF-1) activity by interaction with cooper ions involves FGF-1 dimer formation induced by copper-catalyzed oxidation. J. Biol. Chem. 267:11307–11315 (1992).
Y. M. Torchinsky. Sulfur in Proteins, Pergamon Press, Oxford, 1981.
C. J. Burke, B. L. Steadman, D. B. Volkin, P. K. Tsai, M. W. Bruner, and C. R. Middaugh. The adsorption of proteins to pharmaceutical container surfaces. Int. J. Pharm. 86:89–93 (1992).
H. Mach, C. J. Burke, D. B. Volkin, J. M. Dabora, G. Sanyal, and C. R. Middaugh. Effect of polyanions on the folding and unfolding of acidic fibroblast growth factor. In M. R. Ladisch and A. Bose (eds.), Harnessing Biotechnology for the 21st Century, American Chemical Society, Washington, DC, 1992, pp. 290–293.
H. Mach, D. B. Volkin, C. J. Burke, C. R. Middaugh, R. J. Linhardt, J. Fromm, D. Loganathan, and L. Mattsson. Nature of the interaction of heparin with acidic fibroblast growth factor. Biochemistry, in press (1993).
F. Nachtmann, G. Atzl, and W. D. Roth. Heparin sodium. In K. Florey (ed.), Analytical Profiles of Drug Substances, Vol. 12, Academic Press, New York, 1983, pp. 215–276.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsai, P.K., Volkin, D.B., Dabora, J.M. et al. Formulation Design of Acidic Fibroblast Growth Factor. Pharm Res 10, 649–659 (1993). https://doi.org/10.1023/A:1018939228201
Issue Date:
DOI: https://doi.org/10.1023/A:1018939228201