Abstract
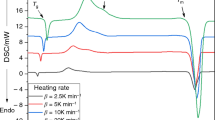
For the first time controlled nucleation and crystallization were studied for 40Fe2O3–20CaO–40SiO2 (wt%) glass which is useful as thermoseeds for hyperthermia of cancer. To investigate the crystallization mechanism of Fe2O3–CaO–SiO2 glass, the Avrami parameter and the activation energy for crystallization were measured by isothermal and non-isothermal processes using classical and differential thermal analysis techniques. Magnetite was the main crystal phase and the maximum nucleation and crystal growth temperatures were 700 and 1000°C, respectively. The value of kinetic parameters such as the Avrami constant, the activation energy, and the frequency factor, determined using isothermal and non-isothermal processes showed excellent agreement. The slopes of the Kissinger, and Matusita and Sakka plots were almost parallel to each other, and, consequently, crystal growth is believed to occur on a fixed number of nuclei, the m values being considered to be the same as the n values. Using m=1.5 and n=1.5, it was found that diffusion-controlled crystal growth with a constant number of nuclei occurred and these result are in excellent agreement with those determined by the classical technique.
Similar content being viewed by others
References
P. F. James, Phys. Chem. Glasses 15 (1974) 95.
E. D. Zanotto and A. Galhardi, J. Non-Cryst. Solids 104 (1988) 73.
A. Morotta, in “Nucleation and Crystallization in Glasses” edited by J. H. Summons, D. R. Uhlmann and G. H. Beall (American Ceramic Society, Columbus, OH, 1982) p. 146.
C. S. Ray and D. E. Day, J. Am. Ceram. Soc. 73 (1990) 439.
A. Morotta, J. Mater. Sci. 16 (1981) 341.
A. A. Luderer, Rad. Res. 94 (1983) 190.
Y. Ebisawa, J. Ceram. Soc. Jpn 99 (1991) 7.
Y. K. Lee and S. Y. Choi, J. Am. Ceram. Soc. 79 (1996) 992.
M. B. Volf, “Chemical Approach to Glass” (Elsevier, Amsterdam, 1984) p. 349.
P. F. James, Phys. Chem. Glasses 15 (1972) 95.
M. Avrami, J. Chem. Phys. 7 (1939) 1103.
T. Ozawa, Polymer 12 (1971) 150.
H. E. Kissinger, Anal. Chem. 29 (1957) 1702.
K. Matusita, J. Mater. Sci. 10 (1975) 961.
K. Matusita and S. Sakka, Phys. Chem. Glasses 20 (1979) 81.
Idem, J. Non-Cryst. Solids 38-39 (1980) 741.
K. Matusita, J. Mater. Sci 19 (1984) 291.
K. Matusita and Komatsu, Thermochim. Acta 88 (1985) 283.
D. W. Henderson, J. Non-Cryst. Solids 30 (1979) 301.
H. Yinnon, ibid. 54 (1983) 253.
M. Matsuura and K. Suzuki, J. Mater. Sci. 14 (1979) 395.
C. S. Ray, J. Am. Ceram. Soc. 74 (1991) 60.
X. J. Xu, ibid, 74 (1991) 909.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LEE, YK., CHOI, SY. Controlled nucleation and crystallization in Fe2O3–CaO–SiO2 glass. Journal of Materials Science 32, 431–436 (1997). https://doi.org/10.1023/A:1018517819830
Issue Date:
DOI: https://doi.org/10.1023/A:1018517819830