Abstract
Background. In several neoplastic diseases, immunosuppression has been shown to correlate with disease stage, progression, and outcome. As the prognosis for metastatic breast cancer is still pessimistic, additional strategies are being sought to improve survival. Local immunosuppression in sentinel node biopsies from 24 evaluable breast cancer patients was studied as a possible way of selecting patients for immunotherapy.
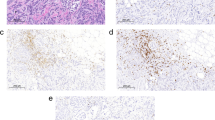
Method. Sentinel node biopsy was performed in 24 out of 25 women operated on for primary breast cancer (one was not evaluable). Specimens were snap-frozen and double-stained for the ζ-chain of the T-cell receptor. The degree of down-regulation of the ζ-chain was evaluated in three different lymph-node areas: primary follicles, secondary follicles, and paracortex.
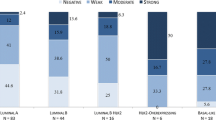
Results. Down-regulation of varying degrees was noted in all 24 sentinel node biopsies. A high degree of down-regulation (more than 50% of T-cells not expressing ζ-chain) was seen in the primary follicles in six patients (25%), in the secondary follicles in 13 patients (72%), and in the paracortex in 19 patients (79%).
Conclusion. Local down-regulation of an immune function parameter was seen in sentinel node biopsies from breast cancer patients. In addition to possible prognostic implications, the sentinel node might be an appropriate location for detecting early-stage immunological down-regulation, which might open a possibility of selecting patients who could benefit from immunotherapy.
Similar content being viewed by others
References
Parkin DM, Pisani P, Ferlay J: Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer 54: 594–606, 1993
Albertini JJ, Lyman GH, Cox C, Yeatman T, Balducci L, Ku N, Shivers S, Berman C, Wells K, Rapaport D, Shons A, Horton J, Greenberg H, Nicosia S, Clark R, Cantor A, Reintgen DS: Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 276(22): 1818–1822, 1996
Morton DL, Wen D-R, Wong JH, Economou JS, Cagle LA, Storm K, Foshag LJ, Cochran AJ: Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 127: 392–399, 1992
Black MM, Kerpe S, Speer FD: Lymph node structure in patients with cancer of the breast. Amer J Path 29: 505–521, 1953
Hutchinson GH, Heinemann D, Symes MO, Williamson RCN: Differential immune reactivity of tumour-intrinsic and peripheral-blood lymphocytes against autoplastic colorectal carcinoma cells. Br J Cancer 44: 396–402, 1981
Miescher S, Whiteside TL, Carrel S, von Fliedner V: Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol 136(5): 1899–1907, 1986
Miescher S, Stoeck M, Qiao L, Barras C, Barrelet L, von Fliedner V: Proliferative and cytolytic potentials of purified human tumor-infiltrating T lymphocytes. Impaired response to mitogen-driven stimulation despite T-cell receptor expression. Int J Cancer 42(5): 659–666, 1988
Vose BM, Moore M: Human tumor-infiltrating lymphocytes: a marker of host response. Sem Hematol 22(1): 27–40, 1985
Cochran AJ, Wen D-R, Stene M, Fairhurst MV, Hoon DSB: Immunobiology of human cutaneous melanoma. Prog Clin Biol Res 256: 597–618, 1988
Head JF, Elliott RL, McCoy JL: Evaluation of lymphocyte immunity in breast cancer patients. Breast Cancer Res Treat 26(1): 77–88, 1993
Garner WL, Minton JP, James AG, Hoffmann CC: Human breast cancer and impaired NK cell function. J Surg Oncol 24(1): 64–66, 1983
Kurt RA, Urba WJ, Smith JW, Schoof DD: Peripheral T lymphocytes from women with breast cancer exhibit abnormal protein expression of several signaling molecules. Int J Cancer 78: 16–20, 1998
Wolfram RM, Budinsky AC, Brodowicz T, Kubista M, Köstler WJ, Kichler-Lakomy C, Hellan M, Kahlhammer G, Wiltschke C, Zielinski CC: Defective antigen presentation resulting from impaired expression of costimulatory molecules in breast cancer. Int J Cancer 88: 239–244, 2000
Whiteside TL: Signaling defects in T lymphocytes of patients with malignancy. Cancer Immunol Immunother 48: 346–352, 1999
Rabinowich H, Banks M, Reichert TE, Logan TF, Kirkwood JM, Whiteside TL: Expression and activity of signaling molecules in T lymphocytes obtained from patients with metastatic melanoma before and after interleukin 2 therapy. Clin Cancer Res 2: 1263–1274, 1996
Reichert TE, Rabinowich H, Johnson JT, Whiteside TL: Immune cells in the tumor microenvironment: mechanisms responsible for signaling and functional defects. J Immunother 21(4): 295–306, 1998
Rabinowich H, Reichert TE, Kashii Y, Gastman BR, Bell MC, Whiteside TL: Lymphocyte apoptosis induced by Fas ligandexpressing ovarian carcinoma cells. Implications for altered expression of T cell receptor in tumor-associated lymphocytes. 101(11): 2579–2588, 1998
Håkansson A, Gustafsson B, Krysander L, Hjelmqvist B, Rettrup B, Håkansson L: On down-regulation of the immune response to metastatic malignant melanoma. Cancer Immunol Immunother 48(5): 253–262, 1999
Kono K, Ressing ME, Brandt RMP, Melief CJM, Potkul RK, Andersson B, Petersson M, Kast WM, Kiessling R: Decreased expression of signal-transducing zeta-chain in peripheral T cells and natural killer cells in patients with cervical cancer. Clin Cancer Res 2: 1825–1828, 1996
Tartour E, Latour S, Mathiot C, Thiounn N, Mosseri V, Joyeux I, D'Enghien CD, Lee R, Debre B, Fridman WH: Variable expression of CD3-ζ-chain in tumor-infiltrating lymphocytes (TIL) derived from renal-cell carcinoma: relationship with TIL phenotype and function. Int J Cancer 63: 205–212, 1995
Rabinowich H, Suminami Y, Reichert TE, Crowley-Nowick P, Bell M, Edwards R, Whiteside TL: Expression of cytokine genes or proteins and signaling molecules in lymphocytes associated with human ovarian carcinoma. Int J Cancer 68: 276–284, 1996
Finke JH, Zea AH, Stanley J, Longo DL, Mizoguchi H, Tubbs RR, Wiltrout RH, O'shea JJ, Kudoh S, Klein E: Loss of T-cell receptor zeta chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res 53(23): 5613–5616, 1993
Zea AH, Curti BD, Longo DL, Alvord WG, Strobl SL, Mizoguchi H, Creekmore SP, O'shea JJ, Powers GC, Urba WJ, Ochoa AC: Alterations in T cell receptor and signal transduction molecules in melanoma patients. Clin Cancer Res 1: 1327–1335, 1995
Kuss I, Saito T, Johnson JT, Whiteside TL: Clinical significance of decreased zeta-chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin Cancer Res 5: 329–334, 1999
Ungefroren H, Voss M, Bernstoff WV, Schmid A, Kremer B, Kalthoff H: Immunological escape mechanisms in pancreatic carcinoma. Ann N Y Acad Sci 880: 243–251, 1999
Nakagomi H, Petersson M, Magnusson I, Juhlin C, Matsuda M, Mellstedt H, Taupin JL, Vivier E, Anderson P, Kiessling R: Decreased expression of the signal-transducing zeta-chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res 53: 5610–5612, 1993
Matsuda M, Petersson M, Lenkei R, Taupin J-L, Magnusson I, Mellstedt H, Anderson P, Kiessling R: Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer 61: 765–772, 1995
Reichert TE, Day R, Wagner EM, Whiteside TL: Absent or low expression of the zeta-chain in T cells at the tumor site correlates with poor survival in patients with oral carcinoma. Cancer Res 58: 5344–5347, 1998
Cochran AJ, Pihl E, Wen D-R, Hoon DSB, Korn EL: Zoned immune suppression of lymph nodes draining malignant melanoma: histologic and immunohistologic studies. J Natl Cancer Inst 78(3): 399–405, 1987
Hoon DSB, Korn EL, Cochran AJ: Variations in functional immunocompetence of individual tumor draining lymph nodes in humans. Cancer Res 47(6): 1740–1744, 1987
Wen D-R, Hoon DSB, Chang C, Cochran AJ: Variations in lymphokine generation by individual lymph nodes draining human malignant tumors. Cancer Immunol Immunother 30(5): 277–282, 1989
Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T: Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci USA 93: 13119–13124, 1996
Aoe T, Okamoto Y, Saito T: Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med 181: 1881–1886, 1995
Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P, Kiessling R: Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol 26: 1308–1313, 1996
Valitutti S, Müller S, Salio M, Lanzavecchia A: Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med 185(10): 1859–1864, 1997
Fu EJ, Arca MJ, Hain JM, Krinock R, Rado J, Cameron MJ, Chang AE, Sondak VK: Tumor-induced suppression of antitumor reactivity and depression of TCR-zeta expression in tumor-draining lymph node lymphocytes: possible relationship to the Th2 pathway. J Immunol 20(2): 111–122, 1997
Jarnicki AG, Fitzpatrick DR, Robinson BWS, Bielefeldt-Ohmann H: Altered CD3 chain and cytokine gene expression in tumor infiltrating T lymphocytes during the development of mesothelioma. Cancer Lett 103: 1–9, 1996
Maccalli C, Pisarra P, Vegetti C, Sensi M, Parmiani G, Anichini A: Differential loss of T cell signaling molecules in metastatic melanoma patients' T lymphocyte subsets expressing distinct TCR variable regions. J Immunol 163: 6912–6923, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schüle, J., Bergkvist, L., Håkansson, L. et al. Down-regulation of the CD3-ζ chain in sentinel node biopsies from breast cancer patients. Breast Cancer Res Treat 74, 33–40 (2002). https://doi.org/10.1023/A:1016009913699
Issue Date:
DOI: https://doi.org/10.1023/A:1016009913699