Abstract
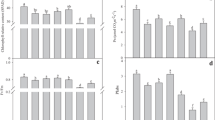
Short-term (one hour) application (painting on surfaces of leaves) of 9 μM GA3 increased net photosynthetic rate (Pn) in broad bean leaves at 31 Pa CO2 and saturating light by more than 20% compared with that of control. The increased Pn was accompanied by an increase in stomatal conductance and a decrease in intercellular CO2 partial pressure. Moreover, the GA3treatment increased the rate of photosynthetic oxygen evolution in isolated broad bean protoplasts to an extent similar to that of leaves. It had little effect on apparent photosynthetic quantum yield and photosynthetic electron transport rate, but could significantly increase carboxylation efficiency in leaves. In consonance with the increase in the carboxylation efficiency, RuBPCase activity and relative content of Rubisco large subunits were also increased by GA3 treatment. Chloramphenicol, an inhibitor of chloroplast protein synthesis, could eliminate the enhancing effect of GA3 on photosynthetic oxygen evolution and relative content of Rubisco large subunits in broad bean protoplasts. Nevertheless, actinomycin D and rifampicin, DNA transcription inhibitors, could not eliminate the enhancement effect of GA3. Similar results were obtained with soybean leaves treated by 90 μM GA3. These results suggest that the increase in leaf net photosynthetic rate caused by GA3 short-term treatment is mainly due to the increases in content and activity of RuBPCase, and that GA3 stimulates the synthesis of Rubisco subunits at translation rather than transcription level.
Similar content being viewed by others
References
Alvim PT (1960) Net assimilation rate and growth behavior of beans as affected by gibberellic acid urea and sugar sprays. Plant Physiol 35: 285–288
Arnon DI (1949) Copper enzymes in isolated chloroplast. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Berry JO, Breiding DE and Klessig DF (1990) Light-mediated control of translational initiation of ribulose-1,5-bisphosphate carboxylase in Amaranth cotyledons. Plant Cell 2: 795–803
Björkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB and Ziegler H (eds) Encyclopedia of Plant Physiology, Physiological Plant Ecology I, Responses to the Physical Environment, Vol 12A, pp 57–107. Springer-Verlag, Berlin
Broughton WJ, Hellmuth EO and Yeung D (1970) Role of glucose in development of the gibberellin response in peas. Biochim Biophys Acta 222: 491–500
Davies PJ (1995) The plant hormones: Their nature, occurrence and functions. In: Davies PJ (ed) Plant Hormones, Physiology, Biochemistry and Molecular Biology, pp 1–12. Kluwer Academic Publishers, Dordrecht, The Netherlands
Deng X-W and Gruissem W (1987) Control of plastid gene expression during development: The limited role of transcriptional regulation. Cell 49: 379–387
Downton WJS, Loveys BR and Grant WJR (1988) Stomatal closure fully accounts for the inhibition of photosynthesis by abscisic acid. New Phytol 108: 263–266
Farquhar GD and Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345
Hames BD and Rickwood D (1990) Gel Electrophoresis of Proteins: A Practical Approach. IRL Press, London
Hayashi T (1961) The effect of gibberellin treatment on the photosynthetic activity of plants. In: IVth International Conference, Plant Growth Regulation, pp 579–588. Iowa State Press, Ames, Iowa
Hong S-S and Xu D-Q (1999) Light-induced increase in initial chlorophyll fluorescence Fo level and the reversible inactivation of PS II reaction centers in soybean leaves. Photosynth Res 61: 269–280
Huber W and Sankhla N (1974) Effect of gibberellic acid on the activities of photosynthetic enzymes and 14CO2 fixation products in leaves of Pennisetum typhoides seedlings. Z Pflanzenphysiol 71: 275–280
Inamine G, Nash B, Weissbach H and Brot N (1985) Light regulation of the synthesis of the large subunit of ribulose-1,5-bisphosphate carboxylase in peas: Evidence for translational control. Proc Natl Acad Sci USA 82: 5690–5694
Keller M, Chan RL, Tessier LH, Weil JH and Imbault P (1991) Posttranslational regulation by light of the biosynthesis of Euglena ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit. Plant Mol Biol 17: 73–82
Kim M, Christopher DA and Mullet JE (1993) Direct evidence for selective modulation of psbA, rpoA, rbcL and 16s RNA stability during barley chloroplast development. Plant Mol Biol 22: 447–463
Klaff P and Gruissem W (1991) Changes in chloroplast mRNA stability during leaf development. Plant Cell 3: 517–529
Laing W, Kreuz K and Apel K (1988) Light-dependent but phytochrome-independent, translational control of the accumulation of the P700 chlorophyll-a protein of Photosystem I in barley (Hordeum vulgare L.). Planta 176: 269–276
Lauerer M, Saftic D, Quick WP, Labate C, Fichtner K, Schulze E-D, Rodermel SR, Bogorad L and Stitt M(1993) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with ‘antisense’ rbcS. VI. Effect on photosynthesis in plants grown at different irradiance. Planta 190: 332–345
Leegood RC and Walker DA (1985) Chloroplasts and protoplasts. In: Coombs J, Hall DO, Long SP and Scurlock JMO (eds) Techniques in Bioproductivity and Photosynthesis, 2nd edn, pp 118–132. Pergamon Press, Oxford, UK
Long SP and Hällgren J-E (1985) Measurement of CO2 assimilation by plants in the field and the laboratory. In: Coombs J, Hall DO, Long SP and Scurlock JMO (eds) Techniques in Bioproductivity and Photosynthesis, pp 62–94. Pergamon Press, Oxford, UK
Malnoe P, Mayfield SP and Rochaix JD (1988) Comparative analysis of the biogenesis of Photosystem II in the wild-type and Y-1 mutant of Chlamydomonas reinhardtii. J Cell Biol 106: 609–616
Marcelle R, Clijsters H, Oben G, Bronchart R and Michel JM (1974) Effects of CCC and GA on photosynthesis of primary bean leaves. In: Science Council of Japan and IPGSA (eds) Plant Growth Substances, Proceedings of the 8th International Conference of Plant Growth Substances, pp 1169–1174. Hirokawa Publishing Company, Tokyo
Nath N and Mishra SD (1990) Stimulation of ribulose-1,5-bisphosphate carboxylase activity in barley flag leaf by plant growth regulators. Photosynthetica 24: 266–269
Oben G and Marcelle R (1975) The effects of CCC and GA on some biochemical and photochemical activities of primary leaves of bean plants. In: Marcelle R (ed) Environmental and Biological Control of Photosynthesis, pp 211–216. Dr W Junk, The Hague
Popova LP, Dimitrova OD and Vaklinova SG (1982) Effect of gibberellin (GA3) on the chlorophyll content, on the intensity of the photosynthetic CO2 fixation and on the activity of carboxylating enzymes in C3 and C4 plants. Dokl Bolg Akad Nauk 35: 797–800
Reid CD, Tissue DT, Fiscus EL and Strain BR (1997) Comparison of spectrophotometric and radioisotopic methods for the assay of Rubisco in ozone-treated plants. Physiol Plant 101: 398–404
Ritchie S and Gilroy S (1998) Gibberellins: Regulating genes and germination. New Phytol 140: 363–383
Schreiber U, Bilger W, Hormann H and Neubauer C (1998) Chlorophyll fluorescence as a diagnostic tool: Basics and some aspects of practical relevance. In: Raghavendra AS (ed) Photosynthesis: A Comprehensive Treatise, pp 320–336. Cambridge University Press, Cambridge, UK
Seemann JR and Berry JA (1982) Interspecific differences in the kinetic properties of RuBP Carboxylase protein. Carnegie Institute Washington Year book 81: 78–83
Stitt M, Quick WP, Schurr U, Schulze E-D, Rodermel SR and Bogorad L (1991) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with ‘antisense’ rbcS. II. Flux-control coefficients for photosynthesis in varying light, CO2, and air humidty. Planta 183: 555–566
Treharne KJ and Stoddart JL (1968) Effects of gibberellin on photosynthesis in red clover (Trifolium pratense L). Nature 220: 457–458
Treharne KJ, Stoddart JL, Pughe J, Paranjothy K and Wareing PF (1970) Effects of gibberellin and cytokinins on the activity of photosynthetic enzymes and plastid ribosomal RNA synthesis in Phaseolus vulgaris L. Nature 228: 129–131
Treharne KJ, Stoddart JL and Hedley CL (1972) Effects of the growth regulant GA3 and CCC on the formation and activity of photosynthetic enzymes in gramineae. In: Forti G, Avron M and Melandri A (eds) Photosynthesis, Two Centuries after Its Discovery by Joseph Priestley, Proceedings of the IInd International Congress on Photosynthesis Res (Stresa 1971), pp 2497–2509. Dr W Junk, The Hague
Von Cammerer S and Farquhar GD (1981) Some relationship between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387
Walker DA (1993) Polarographic measurement of oxygen. In: Hall DO, Scurlock JMO and Bolhar HR (eds) Photosynthesis and Production in a Changing Environment, a Field and Laboratory Manual, pp 268–282. Chapman & Hall, London
Wellburn FAM, Wellburn AR, Stoddart JL and Treharne KJ (1973) Influence of gibberellic and abscisic acids and the growth retardant, CCC, upon plastid development. Planta 111: 337–346
Xu D-Q, Li D-Y, Qiu G-X, Shen Y-K, Huang Q-M, Yang D-D and Beadle CL (1987) Studies on stomatal limitation of photosynthesis in the bamboo (Phyllostachys pubescens) leaves. Acta Phytophysiol Sin 13: 154–160
Xu D-Q, Ding Y and Wu H (1992) Relationship between diurnal variation of photosynthetic efficiency and midday depression of photosynthetic rate in wheat leaves under field conditions. Acta Phytophysiol Sin 18: 279–284
Xue S, Wang P-H, Xu D-Q and Li L-R (1992) Effects of water stress on CO2 assimilation of two winter wheat cultivars with different drought resistance. Acta Phytophysiol Sin 18: 1–7
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, L., Xu, DQ. Stimulation effect of gibberellic acid short-term treatment on leaf photosynthesis related to the increase in Rubisco content in broad bean and soybean. Photosynthesis Research 68, 39–47 (2001). https://doi.org/10.1023/A:1011894912421
Issue Date:
DOI: https://doi.org/10.1023/A:1011894912421