Abstract
The chilling sensitivity of several plant species is closely correlated with the levels of unsaturation of fatty acids in the phosphatidylglycerol (PG) of chloroplast membranes. Plants with a high proportion of unsaturated fatty acids, such as Arabidopsis thaliana, are resistant to chilling, whereas species like squash with only a low proportion are rather sensitive to chilling. The glycerol-3-phosphate O-acyltransferase (GPAT) enzyme of chloroplasts plays an important role in determining the levels of PG fatty acid desaturation.
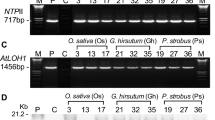
A cDNA for oleate-selective GPAT of Arabidopsis under the control of a maize Ubiquitin promoter was introduced into rice (Oryza sativa L.) using the Agrobacterium-mediated gene transfer method. The levels of unsaturated fatty acids in the phosphatidylglycerol of transformed rice leaves were found to be 28% higher than that of untransformed controls. The net photosynthetic rate of leaves of transformed rice plants was 20% higher than that of the wild type at 17°C. Thus, introduction of cDNA for the Arabidopsis GPAT causes greater unsaturation of fatty acids and confers chilling tolerance of photosynthesis on rice.
Similar content being viewed by others
References
An G, Ebert PR, Mitra A, Ha SB: Binary vectors. In: Gelvin SB, Schilperoort RA (eds) Plant Molecular Biology Manual, pp. 1–19. Kluwer, Dordrecht, Netherlands (1988).
Azcón-Bieto J, Lambers H, Day AD: Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway in leaf respiration. Plant Physiol 72: 598–603 (1983).
Hood EE, Helmer GL, Fraly RT, Chilton MD: The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J Bact 168: 1291–1301 (1986).
Ishii R, Yamaguchi T, Murata Y: On a method for measuring photosynthesis and respiration of leaf slices with an oxygen electrode. Jpn J Crop Sci 46: 53–57 (1977).
Kabaki N, Yoneyama T, Tajima K: Physiological mechanism of growth retardation in rice seedlings as affected by low temperature. Jpn J Crop Sci 51: 82–88 (1982).
Kishitani S, Tsunoda S: Effect of low and high temperature pretreatment on leaf photosynthesis and transpiration in cultivars of Oryza sativa. Photosynthetica 8: 161–167 (1974).
Li T, Lynch DV and Steponkus PL:Molecular species composition of phosphatidylglycerols from rice varieties differing in chilling sensitivity. Cryo-Letters 8: 314–321 (1987).
Mudd JB, Andrews JE, Sparace SA: Phosphatidylglycerol synthesis in chloroplast membranes. Meth Enzymol 148: 338–345 (1987).
Murata N, Sato N, Takahashi N, Hamazaki Y: Composition and positional distributions of fatty acids in phospholipids from leaves of chilling-sensitive and chilling-resistant plants. Plant Cell Physiol 23: 1071–1079 (1982).
Murata N: Molecular species composition of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Cell Physiol 24: 81–86 (1983).
Murata N, Ishizaki-Nishizawa O, Higashi S, Hayashi H, Tasaka Y, Nishida I: Genetically engineered alteration in the chilling sensitivity of plants. Nature 356: 710–713 (1992).
Murry MG and Thompson WF: Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8: 4321–4325 (1980).
Nishiyama I: Cool weather damage of the rice plant. In: Asian Paddy Fields: Their Environmental, Historical, Cultural and Economic Aspects under Various Physical Conditions. Scientific Communication on Problems of the Environment, ICSU (1997).
Nishida I, Tasaka Y, Shiraishi H, Murata N: The gene and the RNA for the precursor to the plastid-located glycerol-3-phosphate acyltransferase of Arabidopsis. Plant Mol Biol 21: 267–277 (1993).
Nishiyama I, Ito N, Hayase H, Satake T: Protecting effect of temperature and depth of irrigation water from sterility caused by cooling treatment at the mitotic stage of rice plants. Proc Crop Sci Jpn 38: 554–555 (Japanese, English summary) (1969).
Satake T: Male sterility caused by cooling treatment at the young microspore stage in rice plants. IX. Revision of the classification and terminology of pollen developmental stages. Proc Crop Sci Soc Jpn 43: 31–35 (1974).
Satake T, Hayase H: Male sterility caused by cooling treatment at the young microspore stage in rice plants. V. Estimation of pollen developmental stage to coolness. Proc Crop Sci Soc Jpn 39: 468–473 (1970).
Sato N, Murata N: Membrane lipids. Meth Enzymol 167: 251–259 (1988).
Terao H, Otani Y, Doi Y, Izumi S: Physiological studies of the rice plant with special reference to the crop failure caused by the occurrence of unseasonable low temperature. VIII. The effect of various low temperature on the panicle differentiation, heading and ripening in the different stages after transplanting to heading. Proc Crop Sci Soc Jpn 13: 317–336 (Japanese) (1941).
Toki S, Takamatsu S, Nojiri C, Ooba S, Anzai H, Iwata M, Christensen AH, Quail PH, Uchimiya H: Expression of a maize ubiquitin gene promoter-bar chimeric gene in transgenic rice plants. Plant Physiol 100: 1503–1507 (1992).
Toriyama S, Hinata K, Nishida I, Murata N: Prominent differences of glycerolipids among anther walls, pollen grains and leaves of rice and maize. Plant Cell Physiol 29: 615–621 (1988).
Tsuchiya T, Toriyama K, Yoshikawa M, Ejiri S, Hinata K: Tapetum-specific expression of the gene for an endo-β-1,3-glucanase causes male sterility in transgenic tobacco. Plant Cell Physiol 36: 487–494 (1995).
Watanabe M, Shiozawa H, Isogai A, Suzuki A, Takeuchi T, Hinata K: Existence of S-glycoprotein-like proteins in anthers of self-incompatible species of Brassica. Plant Cell Physiol 32: 1039–1047 (1991).
Yokoi S, Tsuchiya T, Toriyama K, Hinata K: Tapetum-specific expression of the Osg6B promoter-β-glucuronidase gene in transgenic rice. Plant Cell Rep 16: 363–367 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yokoi, S., Higashi, SI., Kishitani, S. et al. Introduction of the cDNA for shape Arabidopsis glycerol-3-phosphate acyltransferase (GPAT) confers unsaturation of fatty acids and chilling tolerance of photosynthesis on rice. Molecular Breeding 4, 269–275 (1998). https://doi.org/10.1023/A:1009671231614
Issue Date:
DOI: https://doi.org/10.1023/A:1009671231614