Abstract
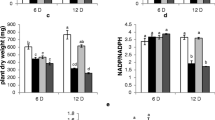
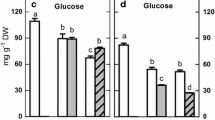
Enhanced amylase activity was observed during a 7-day-growth period in the cotyledons of PEG imposed water stressed chickpea seedlings grown in the presence of GA3 and kinetin, when compared with stressed seedlings. During the first 5 days of seedling growth, the seedlings growing under water deficit conditions as well as those growing in the presence of PGRs had a higher amylase activity in shoots than that of control seedlings. Neither GA3 nor kinetin increased the amylase activity of roots whereas IAA reduced root amylase activity. Activity of acid and alkaline invertases was maximum in shoots and at a minimum in cotyledons. Compared with alkaline invertase, acid invertase activity was higher in all the tissues. The reduced acid and alkaline invertase activities in shoots of stressed seedlings were enhanced by GA3 and kinetin. Roots of stressed seedlings had higher alkaline invertase activity and GA3 and IAA helped in bringing the level near to those in the controls. GA3 and kinetin increased the sucrose synthase (SS) and sucrose phosphate synthase (SPS) activities in cotyledons of stressed seedlings, whereas they brought the elevated level of SPS of stressed roots to near normal level. The higher level of reducing sugars in the shoots of GA3 and kinetin treated stressed seedlings could be due to the high acid invertase activity observed in the shoots, and the high level of bound fructose in the cotyledons of stressed seedlings could be due to the high activity of SPS in this tissue.
Similar content being viewed by others
References
Amzallag GN, Lener HR and Poljakoff-Mayber A (1990) Exogenous ABA as a modulator of the response of sorghum to high salinity. J Exp Bot 541: 1529–1534
Banyal S and Rai VK (1983) Reversal of osmotic stress effects by gibberellic acid in Brassica campestris. Recovery of hypocotyl growth, protein and RNA levels in presence of GA. Physiol Plant 59: 111–114
Barlow FWR, Boersma L and Young JL (1976) Root temperature and soil water potential effects on growth and soluble carbohydrate concentration of corn seedlings. Crop Sci 16: 59–62
Bohnert HJ, Nelson DE and Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7: 1099–1111
Boothby D and Wright STC (1962) Effect of kinetin and other growth regulators on starch degradation. Nature 196: 389–390
Cheikh N and Brenner M (1990) Role of gibberellins in the regulation of carbon metabolism in soybean leaves. Proceedings of the Plant Growth Regulator Society of America. 17th Annual Meeting, St. Paul Minnesota, U.S.A. 5–9 August 1990. Ithaca, New York, USA: Plant Growth Regulator Society of America, pp 151–152
Cheikh N and Brenner ML (1992) Regulation of key enzymes of sucrose biosynthesis in soybean leaves. Effect of dark and light conditions and role of gibberellins and abscisic acid. Plant Physiol 100: 1230–1237
Daie J (1986) Hormone mediated enzyme activity in sugarbeet leaves. Plant Growth Regulation 4: 287–291
Davis BD (1984) Regulation of α-amylase activity in bean stem tissues. Plant Physiol 74: 841–845
Dey PM (1986) Change in the forms of invertases during germination of mungbean seeds. Phytochem 25: 51–53
Dubois M, Gilles KA, Hamilton JK, Rebers PA and Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356
Giaquinta RT (1980) Translocation of sucrose and oligosaccharides. In: Preiss J (ed) The Biochemistry of Plants 3. New York: Academic Press, pp 271–300
Gupta AK, Singh J, Kaur N and Singh R (1993a) Effect of polyethylene glycol induced water stress on germination and reserve carbohydrate metabolism in chickpea cultivars differing in tolerance to water deficit. Plant Physiol Biochem 31: 369–378
Gupta AK, Singh J, Kaur N and Singh R (1993b) Effect of polyethylene glycol induced water stress on uptake, interconversion and transport of sugars in chickpea seedlings. Plant Physiol Biochem 31: 743–747
Guy CL, Huber JLA and Huber SC (1992) Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol 100: 502–508
Hason AD and Hitz WD (1982) Metabolic responses of mesophytes to plant waterdeficit. Ann Rev Plant Physiol 33: 163–203
Heikkila JJ, Rapp J, Schultz GA and Bewley JD (1984) Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic acid and wounding. Plant Physiol 76: 270–274
Hooda RS, Sheoran IS and Singh R (1990) Partitioning and utilization of carbon and nitrogen in nodulated roots and nodules of chickpea (Cicer arietinum) grown at two moisture levels. Ann Bot 65: 111–120
Huber SC, Rogers HH and Mowry FL (1984) Effect of water stress on photosynthesis and carbon partitioning in soybean (Glycine max L. Merr) plants grown in the field at different CO2 levels. Plant Physiol 76: 244–249
Jacobsen JV, Hanson AD and Chandler PN (1986) Water stress enhances the expression of α-amylase gene in barley leaves. Plant Physiol 80: 350–359
Jones MM, Osmond CB and Turner NC (1980) Accumulation of solutes in leaves of sorghum and sunflower in response to water deficits. Aust J Plant Physiol 7: 193–205
Kallarackal J, Orlich G, Schobert C and Komor E (1989) Sucrose transport into phloem of Ricinus communis L. seedlings as measured by the analysis of sieve tube sap. Planta 177: 327–335
Kameli A and Losel DM (1993) Carbohydrates and water status in wheat plants under water stress. New Phytol 125: 609–614
Kaufman MR and Ross KJ (1970) Water potential, temperature and kinetin effects on seed germination in soil and solute systems. Am J Bot 57: 413–419
Kaur S, Gupta AK and Kaur N (1998) Gibberelic acid and kinetin partially reverse the effect of water stress on germination and seedling growth. Plant Growth Regulation 25: 29–33
Kaur S, Gupta AK and Kaur N (1998) Gibberelin A3 reverses the effect of salt stress in chickpea (Cicer arietinum L.) seedlings by enhancing amylase activity and mobilization of starch in cotyledons. Plant Growth Regulation 26: 85–90
Kerr PS, Torres WK and Huber SC (1987) Resolution of two molecular forms of sucrose phosphate synthase from maize, soybean and spinach leaves. Planta 170: 515–519
Kolupaev Yu Ye, Syzonenko SI and Sysoyev LA (1993) Combined effect of auxin and protein synthesis inhibitors on resistance of plant cells to potential lethal osmotic stress. Fiziologiya Biokhimiya Kulturnykh Rastenii 25: 430–436
Koster KL (1991) Glass formation and desiccation tolerance in seeds. Plant Physiol 96: 302–304
Kriedemann P and Beevers H (1967) Sugar uptake and translocation in castor bean seedlings. I. Characteristics of transfer in intact and excised seedlings. Plant Physiol 42: 161–173
Kuhad MS, Sheoran IS and Kumar S (1987) Alleviation and separation of osmotic and ionic effect during germination and early seedling growth in pearl millet by presoaking the seeds with growth regulators. Indian J Plant Physiol 30: 139–142
Lin CC and Kao CH (1995) NaCl stress in rice seedlings: starch mobilization and the influence of GA3 on seedling growth. Botanical Bulletin of Academia Sinica 36: 169–173
Lowry OH, Rosebrough NJ, Farr AL and Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275
Mcrary WL and Slattery M (1945) The colorimetric determination of fructosan in plant material. J Biol Chem 157: 161–167
Meyer RF and Boyer JS (1981) Osmoregulation, solute distribution and growth in soybean seedlings having low water potentials. Planta 151: 482–489
Miyamoto K and Kamisaka S (1990) Effect of gibberellic acid in epicotyl growth and carbohydrate distribution in derooted Pisum sativum cuttings with or without cotyledons. Physiol Plant 80: 357–364
Miyamoto K, Ueda J and Kamisaka S (1993) Gibberellin enhanced sugar accumulation in growing subhooks of etiolated Pisum sativum seedlings. Effects of gibberellic acid, indole acetic acid and cycloheximide on invertase activity, sugar accumulation and growth. Physiol Plant 88: 301–306
Monerri C, Garcia-Luis A and Guardiola JL (1986) Sugar and starch changes in pea cotyledons during germination. Physiol Plant 67: 49–54
Munns R, Brady CJ and Barlow EWR (1979) Solute accumulation in the apex and leaves of wheat during water stress. Aust J Plant Physiol 6: 379–389
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–497
Naylor AW (1984) Hormonal regulation of development. II. The function of hormones from the level of the cell to whole plant. In: Scot TK (ed) Encyclopedia of Plant Physiology, New Series, Vol. 10. Berlin: Springer Verlag, pp 180–185
Nelson N (1944) A photometric adaptation of Somogyi method for the determination of glucose. J Biol Chem 153: 375–380
Parker J (1972) Protoplasmic resistance to water deficits. In: Kozlowski TT (ed) Water Deficits and Plant Growth, Vol. 3. New York/London: Academic Press, pp 125–176
Quick P, Siegle G, Neuhaus E, Feil R and Stitt M (1989) Short term water stress leads to a stimulation of sucrose synthesis by activating sucrose phosphate synthase. Planta 177: 535–546
Rudolph AS, Crowe JH and Crowe LM (1986) Effects of three stabilizing agents-proline, betaine and trehalose-on membrane phospholipids. Arch Biochem Biophys 245: 134–143
Santarius KA (1973) The protective effect of sugars on chloroplast membranes during temperature and water stress and its relationship to frost, desiccation and heat resistance. Planta 113: 105–114
Seitz K and Lang A (1968) Invertase activity and cell growth in lentil epicotyls. Plant Physiol 43: 1075–1082
Sheoran IS (1980) Changes in amylase during germination and early seedling growth of mungbean (Vigna radiata L.Wilczek) under different salts. Indian J Plant Physiol 23: 168–173
Sheoran IS, Kaur N and Singh R (1988) Nitrogen fixation and carbon metabolism in nodules of pigeonpea (Cajanus cajan L.) under drought stress. J Plant Physiol 132: 480–483
Spyropoulos CG (1982) Control of sucrose metabolism in polyethylene glycol stressed carob (Ceratonia siliqua L.) young seedlings. The role of sucrose. J Exp Bot 33: 1210–1219
Timpa JD, Burke JJ, Quisenberry JE and Wendt CW (1986) Effects of water stress on the organic acid and carbohydrate composition of cotton plants. Plant Physiol 82: 724–728
Todd GW (1972) Water deficit and enzyme activity. In: Kozlowski TT (ed) Water Deficits and Plant Growth, Vol. 3. New York: Academic Press, pp 177–216
Zelitch I (1982) The close relationship between net photosynthesis and crop yield. Bioscience 32: 792–802
Rights and permissions
About this article
Cite this article
Kaur, S., Gupta, A.K. & Kaur, N. Effect of GA3, kinetin and indole acetic acid on carbohydrate metabolism in chickpea seedlings germinating under water stress. Plant Growth Regulation 30, 61–70 (2000). https://doi.org/10.1023/A:1006371219048
Issue Date:
DOI: https://doi.org/10.1023/A:1006371219048