Abstract
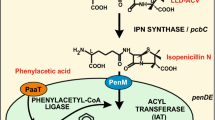
A proper description of the biosynthesis of fungal β-lactam antibiotics requires detailed knowledge of the cell biology of the producing organisms. This involves a delineation of the compartmentalization of the biosynthetic pathways, and of the consequential transport steps across the cell-boundary plasma membrane and across organellar membranes. Of the enzymes of the penicillin biosynthetic pathway in Penicillium chrysogenum and Aspergillus nidulans, δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine synthetase (ACVS) and isopenicillin N synthase (IPNS) probably have a cytosolic location. Acyl-coenzyme A:isopenicillin N acyltransferase (IAT) is located in microbodies. Of the two enzymes that may be involved in activation of the side chain, acetyl-coenzyme A synthetase (ACS) is located in the cytosol, and phenylacetyl-coenzyme A ligase (PCL) is probably located in the microbody. All enzymes of the cephalosporin biosynthesis pathway in Cephalosporium acremonium probably have a cytosolic location. The vacuole may play an ancillary role in the supply of precursor amino acids, and in the storage of intermediates. The distribution of precursors, intermediates, end- and side-products, the transport of nutrients, precursors, intermediates and products across the plasma membrane, and the transport of small solutes across organellar membranes, is discussed. The relevance of compartmentalization is considered against the background of recent biotechnological innovations of fungal β-lactam biosynthesis pathways.
Similar content being viewed by others
References
Abraham EP, Huddleston JA, Jayatilake GS, O'Sullivan J & White RL (1981) Conversion of δ-(L-α-amino-adipyl)-L-cysteinyl-D-valine to isopenicillin N in cell-free extracts of Cephalosporium acremonium. In: Gregory (Ed) Recent Advances in the Chemistry of β-Lactam Antibiotics (pp 125–134). Royl Soc Chem, London.
Adlard MW, Gordon BM, Keshavarz T, Bucke T, Lilly MD, Bull AT & Holt G (1991) An HPLC procedure for monitoring penicillin G fermentations. Biotechnol Techn 5: 121–126.
Affenzeller K & Kubicek CP (1991) Evidence for a compartmentation of penicillin biosynthesis in a high-and a low-producing strain of Penicillium chrysogenum. J Gen Microbiol 137: 1653–1660.
Aharonowitz Y, Cohen G & Martín JF (1992) Penicillin and cephalosporin biosynthesis genes: structure, organization, regulation, and evolution. Annu Rev Microbiol 46: 461–495.
Aharonowitz Y, Bergmeyer J, Cantoral JM, Cohen G, Demain AL, Fink U, Kinghorn J, Kleinkauf H, MacCabe A, Palissa H, Pfeifer E, Schwecke T, van Liempt H, von Döhren H, Wolfe S & Zhang J (1993a) δ-(L-α-amino-adipyl)-L-cysteinyl-D-valine synthetase, the multienzyme integrating the four primary reactions in β-Lactam biosynthesis, as a model peptide synthetase. Bio/Technology 11: 807–810.
Aharonowitz Y, Av-Gay Y, Schreiber R & Cohen G (1993b) Characterization of a broad-range disulfide reductase from Streptomyces clavuligerus and its possible role in β-lactam antibiotic biosynthesis. J Bacteriol 175: 623–629.
Alonso MJ, Bermejo F, Reglero A, Fernández-Cañón JM, de Buitrago GG & Luengo JM (1988) Enzymatic synthesis of penicillins. J Antibiot 41: 1074–1084.
Alvarez E, Cantoral JM, Barredo JL, Díez B & Martín JF (1987) Purification to homogeneity and characterization of acyl coenzyme A:6-aminopenicillanic acid acyltransferase of Penicillium chrysogenum. Antimicrob Agents Chemother 31: 1675–1682.
Alvarez E, Meeschaert B, Montenegro E, Gutiérrez S, Díez B, Barredo JL & Martín JF (1993) The isopenicillin-N acyltransferase of Penicillium chrysogenum has isopenicillin-N amidohydrolase, 6-aminopenicillanic acid acyltransferase and penicillin amidase activities, all of which are encoded by the single penDE gene. Eur J Biochem 215: 323–332.
Alvi KA, Reeves CD, Peterson & Lein J (1995) Isolation of a new cephem compound from Penicillium chrysogenum strains expressing deacetoxycephalosporin C synthase activity. J Antibiot 48: 338–340.
André B (1995) An overview of membrane transport proteins in Saccharomyces cerevisiae. Yeast 11: 1575–1611.
Anraku Y (1996) Structure and function of the yeast vacuolar membrane H+-ATPase. In: Konings WN, Kaback HR & Lolkema JS (Eds) Handbook of Biological Physics (pp 93–109). Elsevier, Cambridge.
Aplin RT, Baldwin JE, Cole SCJ, Sutherland JD & Tobin MB (1993) On the production of α, β-heterodimeric acyl-coenzyme A:isopenicillin N-acyltransferase of Penicillium chrysogenum. FEES Lett 319: 166–170.
Arst Jr HN (1968) Genetic analysis of the first steps of sulphate metabolism in Aspergillus nidulans. Nature 219: 268–270.
Baldwin JE, Keeping JW, Singh PD & Vallejo CA (1981) Cell-free conversion of isopenicillin N into deacetoxy-cephalosporin C by Cephalosporium acremonium mutant M-0198. Biochem J 194: 649–651.
Baldwin JE, Gagnon J & Ting H-H (1985a) N-terminal amino acid sequence and some properties of isopenicillin-N synthetase from Cephalosporium acremonium. FEBS Lett 188: 253–256.
Baldwin JE, Moroney SE & Ting H-H (1985b) A coupled assay for isopenicillin-N synthetase. Anal Biochem 145: 183–187.
Baldwin JE, Killin SJ, Pratt AJ, Sutherland JD, Turner NJ, Crabbe JC, Abraham EP & Willis AC (1987) Purification and characterization of cloned isopenicillin N synthetase. J Antibiot 40: 652–659.
Baldwin JE, Bird JW, Field RA, O'Callaghan NM & Schofield CJ (1990) Isolation and partial characterisation of ACV synthetase from Cephalosporium acremonium and Streptomyces clavuligerus. J Antibiot 43: 1055–1057.
Baldwin JE, Bird JW, Field RA, O'Callaghan NM, Schofield CJ & Willis AC (1991) Isolation and partial character-isation of ACV synthetase from Cephalosporium acremonium and Streptomyces clavuligerus: evidence for the presence of phosphopantothenate in ACV synthetase. J Antibiot 44: 241–248.
Baldwin JE, Shiau C-Y, Byford MF & Schofield CJ (1994) Substrate specificity of δ-L-(α-amino-adipoyl)-L-cysteinyl-D-valine synthetase from Cephalosporium acremonium: demonstration of the structure of several unnatural tripeptide products. Biochem J 301: 367–372.
Banko G, Demain AL & Wolfe S (1987) δ-(L-α-Aminoadipyl)-L-cysteinyl-L-valine synthetase (ACV synthetase): a multifunctional enzyme with broad substrate specificity for the synthesis of penicillin and cephalosporin precursors. J Am Chem Soc 109: 2858–2860.
Barredo JL, Cantoral JM, Alvarez E, Díez B & Martín JF (1989a) Cloning, sequence analysis and transcriptional study of the isopenicillin N synthase of Penicillium chrysogenum AS-P-78. Mol Gen Genet 216: 91–98.
Barredo JL, van Solingen P, Díez B, Alvarez E, Cantoral JM, Kattevilder A, Smaal EB, Groenen MAM, Veenstra AE& Martín JF (1989b) Cloning and characterization of the acyl-coenzyme A: 6-amino-penicillanic-acid-acyltransferase gene of Penicillium chrysogenum. Gene 83: 291–300.
Barrios-González J, Montenegro E & Martín JF (1993) Penicillin production by mutants resistant to phenylacetic acid. J Ferment Bioeng 76: 455–458.
Bates WK, Hedman SC & Woodward DO (1967) Comparative inductive responses of two β-galactosidases of Neurospora. J Bacteriol 93: 1631–1637.
Benko PV, Wood TC & Segel IH (1969) Multiplicity and regulation of amino acid transport in Penicillium chrysogenum. Arch Biochem Biophys 129: 498–508.
Bennet MK & Scheller RH (1993) The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA 90: 2559–2563.
Berteaux-Lecellier V, Picard M, Thompson-Coffe C, Zickler D, Panvier-Adoutte A & Simonet J-M (1995) A non-mammalian homolog of the PAF1 gene (Zellweger syndrome) discovered as a gene involved in cayogamy in the fungus Podospora anserina. Cell 81: 1043–1051.
Bhattacharjee JK (1985) α-Aminoadipate pathway for the biosynthesis of lysine in lower eukaryotes. Crit Rev Microbiol 12: 131–151.
Bhattacharjee JK (1992) Evolution of α-aminoadipate pathway for the synthesis of lysine in fungi. In: Mortlock RP (Ed) The Evolution of Metabolic Function (pp 47–80). CRC Press, Boca Raton, FL.
Bovenberg RAL, Vollebregt AWH & Schipper D (1988) Formation of cephalosporins by Penicillium chrysogenum. In: Martín JF & Gutiérrez S (Eds) Fungal Genetics — Fourth European Conference on Fungal Genetics (p 201). Universidad de León, León.
Bowman EJ & Bowman BJ (1982) Identification and properties of an ATPase in vacuolar membranes of Neurospora crassa. J Bacteriol 151: 1326–1337.
Bowman BJ & Bowman EJ (1996) Mitochondrial and vacuolar AT-Pases. In: Brambl R & Marzluf GA (eds) The Mycota III (pp 57–83). Springer-Verlag, Berlin Heidelberg
Bradfield G, Somerfield P, Meyn T, Holby M, Badcock D, Bradley D & Segel IH (1970) Regulation of sulfate transport in filamentous fungi. Plant Physiol 46: 720–727.
Brakhage AA (1997) Molecular regulation of penicillin biosynthesis in Aspergillus (Emericella) nidulans. FEMS Microbiol Lett 148: 1–10.
Brakhage AA (1998) Molecular regulation of β-lactam biosynthesis in filamentous fungi. Microbiol Mol Biol Rev 62: 547–585.
Brakhage AA & Turner G (1992) L-Lysine repression of penicillin biosynthesis and expression of penicillin biosynthesis genes acvA and ipnA in Aspergillus nidulans. FEMS Microbiol Lett 98: 123–128.
Brakhage AA & Turner G (1995) Biotechnological genetics of antibiotic biosynthesis. In: Kück U (Ed) The Mycota II (pp 263–285). Springer-Verlag, Berlin.
Brakhage AA, Browne P & Turner G (1992) Regulation of Aspergillus nidulans penicillin biosynthesis and penicillinbiosynthesis genes acvA and ipnA by glucose. J Bacteriol 174: 3789–3799.
Brodelius P & Pedersen H (1993) Increasing secondary metabolite production in plant-cell culture by redirecting transport. Trends Biotechnol 11: 30–36.
Brownlee AG & Arst HN Jr (1983) Nitrate uptake in Aspergillus nidulans and involvement of the third gene of the nitrate assimilation gene cluster. J Bacteriol 155: 1138–1146.
Brundidge SP, Gaeta FCA, Hook DJ, Sapino C, Blander RP & Morin RB (1980) Association of 6-oxo-piperidine-2-carboxylic acid with penicillin V production in Penicillium chrysogenum fermentations. J Antibiot 33: 1348–1351.
Brunner R & Röhr M (1975) Phenacyl:coenzyme A ligase. Methods Enzymol 43: 476–481.
Bryant NJ & Stevens TH (1998) Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev 62: 230–247.
Bun Ya M, Nishimura M, Harashima S & Oshima Y (1991) The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol 11: 3229–3238.
Burgstaller W (1997) Transport of small ions and molecules through the plasma membrane of filamentous fungi. Crit Rev Microbiol 23: 1–46.
Caddick MX, Brownlee AG & Arst Jr HN (1986) Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Mol Gen Genet 203: 346–353.
Cantwell C, Beckamnn R, Whiteman P, Queener SW & Abraham EP (1992) Isolation of deacetoxycephalosporinC from fermentation broths of Penicillium chrysogenum transformants: construction of a new fungal biosynthetic pathway. Proc R Soc Lond B 248: 283–289.
Carr LG, Skatrud PL, Scheetz ME, Queener SW & Ingolia TD (1986) Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene 48: 257–266.
Carson DB & Cooney JJ (1990) Microbodies in fungi: a review. J Industr Microbiol 6: 1–18.
Cartier N, Lopez J, Moullier P, Rocchiccioli F, Rolland M-O, Jorge P, Mossere J, Mandel J-L, Bougnères P-F, Danos O & Aubourg P (1995) Retroviral-mediated gene transfer corrects very-long-chain fatty acid metabolism in adrenoleukodystrophy fibroblasts. Proc Natl Acad Sci USA 92: 1674–1678.
Casqueiro J, Gutíerrez S, Bañuelos O, Fierro F, Hijarrubia MJ, Campoy S, Velasco J & Martín JF (1988a) Cloning and characterization of the lys2 gene of Penicillium chrysogenum encoding α-aminoadipate reductase: identification of a functional reduction domain. In: Martín JF & Gutiérrez S (Eds) Fungal Genetics — Fourth European Conference on Fungal Genetics (p 190). Universidad de León, León.
Casqueiro J, Gutíerrez S, Bañuelos O, Fierro F, Velasco J & Martín JF (1988b) Characterization of the lys2 gene of Penicillium chrysogenum encoding α-aminoadipate reductase. Mol Gen Genet 259: 549–556.
Cassio F & Leao C (1991) Low-and high-affinity transport systems for citric acid in the yeast Candida utilis. Appl Environ Microbiol 57: 3623–3628.
Cassio F & Leao C (1993) A comparative study on the transport of L levo malic acid and other short-chain carboxylicacids in the yeast Candida utilis: Evidence for a general organic acid permease. Yeast 9: 743–752.
Castro JM, Liras P, Laíz L, Cortés J & Matín JF (1988) Purification and characterization of the isopenicillin N synthase of Streptomyces lactamdurans. J Gen Microbiol 134: 133–141.
Chang Y-D & Dickson RC (1988) Primary structure of the lactose permease gene from the yeast Kluyveromyces lactis. J Biol Chem 263: 16696–16703.
Cherest H, Davidian J-C, Thomas D, Benes V, Ansorge W & Surdin-Kerjan Y (1997) Molecular characterization of two high affinity sulfate transporters in Saccharomyces cerevisiae. Genetics 145: 627–635.
Christensen LH, Mandrup G, Nielsen J & Villadsen J (1994a) A robust LC method for measurement of medium components during penicillin fermentations. Anal Chim Acta 296: 51–62.
Christensen LH, Nielsen J & Villadsen J (1994b) Degradation of penicillin V in fermentation media. Biotechnol Bioeng 44: 165–169.
Christensen LH, Henriksen CM, Nielsen J, Villadsen J & Egel-Mitani M (1995) Continuous cultivation of Penicillium chrysogenum. Growth on glucose and penicillin production. J Biotechnol 42: 95–107.
Chu YW, Renno D & Saunders G (1997) Extracellular pH affects regulation of the pcbAB gene in Penicilliumchrysogenum. Appl Microbiol Biotechnol 47: 250–254.
Cohen G, Argaman A, Schreiber R, Mislovati M & Aharonowitz Y (1994) The thioredoxin system of Penicillium chrysogenum and its possible role in penicillin biosynthesis. J Bacteriol 176: 973–984.
Conibear E & Stevens TH (1995) Vacuolar biogenesis in yeast: sorting out the sorting proteins. Cell 83: 513–516.
Connerton IF, Fincham JRS, Sanderman RA & Hynes MJ (1990) Comparison and cross-species expression of the acetyl-CoA synthetase genes of the ascomycete fungi, Aspergillus nidulans and Neurospora crassa. Mol Microbiol 4: 451–160.
Coque JJR, Martín JF, Calzada JG & Liras P (1991) The cephamycin biosynthetic genes pcbAB, encoding a large multidomain peptide synthetase, and pcbC of Nocardia lactamdurans are clustered together in an organization different from the same genes in Acremonium chrysogenum and Penicillium chrysogenum. Mol Microbiol 5: 1125–1133.
Coque JJ, Liras P & Martín JF (1993a) Genes for a β-lactamase, a penicillin-binding protein and a transmembrane protein are clustered with the cephamycin biosynthetic genes in Nocordia lactamdurans. EMBO J 12: 631–639.
Coque JJR, Martín JF & Liras P (1993b) Characterization and expression in Streptomyces lividans of cefD and cefE genes from Nocardia lactamdurans: The organization of the cephamycin gene cluster differs from that in Streptomyces clavuligerus. Mol Gen Genet 236: 453–158.
Coque JJR, de la Fuente JL, Liras P & Martín JF (1996a) Overexpression of the Nocardia lactamdurans α-amino-adipyl-cysteinyl-valine synthetase in Streptomyces lividans. The purified multienzyme uses cystathione and 6-oxopiperidine 2-carboxylate as substrates for synthesis of the tripeptide. Eur J Biochem 242: 264–279.
Coque JJR, Enguita FJ, Cardoza RE, Martín JF & Liras P (1996b) Characterization of the cefF gene of Nocardialactamdurans encoding a 3′-methylcephem hydroxylase different from the 7-cephem hydroxylase. Appl Microbiol Biotechnol 44: 605–609.
Corte-Real M & Leao C (1990) Transport of malic acid and other dicarboxylic acids in the yeast Hansenula anomala. Appl Environ Microbiol 56: 1109–1113.
Cortés J, Martín JF, Castro JM, Laíz L & Liras P (1987) Purification and characterization of a 2-oxoglutarate linked ATP independent deacetoxycephalosporin C synthase of S. lactamdurans. J Gen Microbiol 133: 3165–3174.
Crawford L, Stepan AM, McAda PC, Rambosek JA, Conder MJ, Vinci VA & Reeves CD (1995) Production of cephalosporin intermediates by feeding adipic acid to recombinant Penicillium chrysogenum strains expressing ring expansion activity. Bio/Technology 13: 58–62.
Crueger W & Crueger A (1990) Biotechnology: a Textbook of Industrial Microbiology (2nd ed) (pp 229–273). Sinauer Associates, Sunderland, MA.
Cui Z, Hirata D, Tsuchiya E, Osada H & Miyakawa T (1996) The multidrug resistance-associated protein (MRP) subfamily (Yrs1/Yor1) of Saccharomyces cerevisiae is important for the tolerance to a broad range of organic anions. J Biol Chem 271: 14712–14716.
Cundliffe E (1989) How antibiotic-producing organisms avoid suicide? Annu Rev Microbiol 43: 207–233.
Cuppoletti J & Segel IH (1975) Kinetics of sulfate transport by Penicillium notatum. Interactions of sulfate, protons,and calcium. Biochemistry 14: 4712–4718.
Daniel H (1996) Function and molecular structure of brush border membrane peptide/H+ symporters. J Membr Biol 154: 197–203.
Decottignies A & Goffeau A (1997) Complete inventory of the yeast ABC proteins. Nat Genet 15: 137–145.
Deeley RG & Cole SPC (1997) Function, evolution and structure of multidrug resistance protein (MRP). Semin Cancer Biol 8: 193–204.
de Hoop MJ & AB G (1992) Import of proteins into peroxisomes and other microbodies. Biochem J 286: 657–669.
del Carmen Mateos R, & Sánchez S (1990) Transport of neutral amino acids and penicillin formation in Penicillium chrysogenum. J Gen Microbiol 136: 1713–1716.
Del Sorbo G, Andrade AC, van Nistelrooy JGM, van Jan JAL, Balzi E & de Waard MA (1997) Multidrug resistance in Asperglllus nidulans involves novel ATP-binding cassette transporters. Mol Gen Genet 254: 417–426.
De Lucas JR, Valenciano S, Domínguez AL, Turner G & Laborada F (1997) Characterization of oleate non-utilizing mutants of Aspergillus nidulans isolated by the 3-amino-1,2,4-triazole positive selection method. Arch Microbiol 168: 504–512.
De Lucas JR, Valenciano S, Domínguez AL & Laborada F (1998) Characterization of oleate non-utilizing mutants of Aspergillus nidulans isolated by the 3-amino-1,2,4-triazole positive selection method. In: Martín JF & Gutiérrez S (Eds) Fungal Genetics — Fourth European Conference on Fungal Genetics (p 63). Universidad de León, León.
Demain AL (1984) Biology of antibiotic formation. In: Vandamme EJ (Ed) Biotechnology of Industrial Antibiotics (pp 33–42). Marcel Dekker, New York.
Demain AL (1991) Biosynthesis and metabolic regulation of β-lactam antibiotics. In: Kleinkauf H & von Döhren H (Eds) 50 Years of Penicillin Application — History and Trends (pp 77–96). Public, Czech Republic.
de Nobel JG (1991) Permeability of the cell wall in Saccharomyces cerevisiae. PhD thesis. Univ of Amsterdam, Amsterdam.
de Nobel JG & Barnett JA (1991) Passage of molecules through yeast cell walls: a brief essay-review. Yeast 7: 313–23.
de Zoysa PA & Connerton IF (1994) The function and specificity of the C-terminal tripeptide glyoxysomal targeting signal in Neurospora crassa. Curr Genet 26: 430–437.
Dickson RC & Barr K (1983) Characterization of lactose transport in Kluyveromyces lactis. J Bacteriol 154: 1245–1251.
Díez B, Gutiérrez S, Barredo JL, van Solingen P, van der Voort LHM & Martín JF (1990) The cluster of penicillin biosynthetic genes. Identification and characterization of the pcbAB gene encoding the α-aminoadipyl-cysteinyl-valine synthetase and linkage to the pcbC and penDE genes. J Biol Chem 265: 16358–16365.
Díez B, Mellado E, Rodriguez M, Fouces R & Barredo J-L (1997) Recombinant microorganisms for industrial production of antibiotics. Biotechnol Bioeng 55: 216–226.
Döring F, Michel T, Rösel A, Nickolaus M & Daniel H (1998) Expression of the mammalian renal peptide transporter PEPT2 in the yeast Pichia pastoris and applications of the yeast system for functional analysis. Mol Membr Biol 15: 79–88.
Dotzlaf JE & Yeh W-K (1987) Copurification and characterization of deacetoxycephalosporin C synthetase/hydroxylase from Cephalosporium acremonium. J Bacteriol 169: 1611–1618.
Douma AC, Veenhuis M, Sulter & Harder W (1987) A protontranslocating adenosine triphosphatase is associated with the peroxisomal membrane of yeasts. Arch Microbiol 147: 42–47.
Douma AC, Veenhuis M, Sulter GJ, Waterham HR, Verheyden K, Mannaerts GP & Harder W (1990) Permeability properties of peroxisomal membranes from yeasts. Arch Microbiol 153: 490–495.
Elgersma Y & Tabak HF (1996) Proteins involved in peroxisome biogenesis and functioning. Biochim Biophys Acta 1286: 269–283.
Elgersma Y, van Roermund CWT, Wanders RJA & Tabak HF (1995) Peroxisomal and mitochondrial carnitine acetyltransferases of Saccharomyces cerevisiae are encoded by a single gene. EMBO J 14: 3472–3479.
Elgersma Y, Vos A, van den Berg M, van Roermund CWT, van der Sluijs P, Distel B & Tabak HF (1996) Analysis of the carboxyterminal peroxisomal targeting signal 1 in a homologous context in Saccharomyces cerevisiae. J Biol Chem 271: 26375–26382.
Elskens MT, Jaspers C & Penninckx MJ (1991) Glutathione as an endogenous sulphur source in the yeast Saccharomyces cerevisiae. J Gen Microbiol 137: 637–644.
Emri T, Pócsi I & Szentirmai A (1997) Phenoxyacetic acid induces glutathione-dependent detoxification and depletes the glutathione pool in Penicillium chrysogenum. J Basic Microbiol 37: 181–186.
Emri T, Pócsi I & Szentirmai A (1998) Changes in the glutathione (GSH) metabolism of Penicillium chrysogenum grown on different nitrogen, sulphur and carbon sources. J Basic Microbiol 38: 3–8.
Erdélyi A, Nyiri L & Lengyel ZL (1966) The change of peroxidase and catalase enzyme activity in submerged cultures of Penicillium chrysogenum. Z Allg Mikrobiol 6: 329–334.
Erickson RC & Bennett RE (1965) Penicillin acylase activity of Penicillium chrysogenum. Appl Microbiol 11: 738–742.
Eriksen SH, Jensen B, Schneider I, Kaasgaard S & Olsen J (1994) Utilization of side-chain precursors for penicillin biosynthesis in a high-producing strain of Penicillium chrysogenum. Appl Microbiol Biotechnol 40: 883–887.
Eriksen SH, Jensen B, Schneider I, Kaasgaard S & Olsen J (1995) Uptake of phenoxyacetic acid by Penicilliumchrysogenum. Appl Microbiol Biotechnol 42: 945–950.
Esmahan C, Alvarez E, Montenegro E & Martín JF (1994) Catabolism of lysine in Penicillium chrysogenum leads to formation of 2-aminoadipic acid, a precursor of penicillin biosynthesis. Appl Environ Microbiol 60: 1705–1710.
Espeso EA & Peñalva MA (1992) Carbon catabolite repression can account for the temporal pattern of expression of a penicillin biosynthetic gene in Aspergillus nidulans. Mol Gen Genet 6: 1457–1465.
Espeso EA, Tilburn J, Arst HN Jr & Peñalva MA (1993) pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J 12: 3947–3956.
Fantes PA & Roberts CF (1973) β-Galactosidase activity and lactose utilization in Aspergillus nidulans. J Gen Microbiol 77: 471–186.
Fawcett P & Abraham EP (1975) δ-(L-α-Aminoadipyl)-L-cysteinyl-D-valine synthetase. Methods Enzymol 43: 471–473.
Fawcett P & Abraham EP (1976) Biosynthesis of penicillins and cephalosporins. Biosynthesis 4: 248–265.
Félix HR, Nüesch J & Wehrli W (1980) Investigation of the two final steps on the biosynthesis of cephalosporin C using permeabilized cells of Cephalosporium acremonium. FEMS Microbiol Lett 8: 55–58.
Félix HR, Peter HH & Treichler HJ (1981) Microbiological ring expansion of penicillin N. J Antibiot 34: 567–575.
Feng B, Friedlin E & Marzluf GA (1994) A reporter gene analysis of penicillin biosynthesis gene expression in Penicillium chrysogenum and its regulation by nitrogen and glucose catabolite repression. Appl Environ Microbiol 60: 4432–4439.
Fernández FJ, Gutiérrez S, Velasco J, Montenegro E, Marcos AT & Martín JF (1994) Molecular characterization of three loss-of-function mutations in the isopenicillin N-acetyltransferase gene (penDE) of Penicillinium chrysogenum. J Bacteriol 176: 4941–4948.
Fernández FJ, Velasco J, Montenegro E, Gutíerrez S, Fierro F & Martín JF (1998) The isopenicillin N acyl-transferases of Aspergillus nidulans and Penicillium chrysogenum differ in their ability to self-process the 40-kDa proacyltransferase and to maintain the αβ heterodimer in an undissociated form. In: Martín JF & Gutiérrez S (Eds) Fungal Genetics — Fourth European Conference on Fungal Genetics (p 192). Universidad de León, León.
Fernández-Cañón JM & Luengo JM (1997) Study of the phenylacetic acid uptake system of Aspergillus nidulans and demonstration that it is under a creA-independent model of catabolic repression which seems to be mediated by acetyl-CoA. J Antibiot 50: 45–52.
Fernández-Cañón JM & Peñalva MA (1995a) Molecular characterization of a gene encoding homogentisate dioxygenase from Aspergillus nidulans and identification of its human and plant homologues. J Biol Chem 270: 21199–21205.
Fernández-Cañón JM & Peñalva MA (1995b) Overexpression of two penicillin structural genes in Aspergillus nidulans. Mol Gen Genet 246: 110–118.
Fernández-Cañón JM, Reglero A, Martínez-Blanco H & Luengo JM (1989a) I. Uptake of phenylacetic acid by Penicillium chrysogenum Wis54–1255: A critical regulatory point in benzylpenicillin biosynthesis. J Antibiot 42: 1398–1409.
Fernández-Cañón JM, Reglero A, Martínez-Blanco H, Ferrero MA & Luengo JM (1989b) II. Phenylacetic acid transport system in Penicillium chrysogenum Wis 54–1255: molecular specificity of its induction. J Antibiot 42: 1410–1415.
Ferrero MA, Reglero A, Martín-Villacorta J, Fernández-Cañón JM & Luengo JM (1990) Biosynthesis of benzyl-penicillin (G), phenoxymethylpenicillin (V) and octanoylpenicillin (K) from glutathione S-derivatives. J Antibiot 43: 684–691.
Friedrich CG & Demain AL (1978) Uptake and metabolism of α-aminoadipic acid by Penicillium chrysogenum Wis54–1255. Arch Microbiol 119: 43–47.
Fujisawa Y & Kanzaki T (1975) Role of acetyl-CoA:deacetoxycephalosporin C acetyltransferase in cephalosporin C biosynthesis by Cephalosporium acremonium. Agric Biol Chem 39: 2043–2048.
Fujisawa Y, Shirafuji H, Kida M, Nara K, Yoneda M & Kanzaka T (1973) New findings on CPC biosynthesis. Nature New Biol 246: 154.
Fujisawa Y, Shirafuji H. Kida M, Nara K, Yoneda M & Kanzaki T (1975) Accumulation of deacetylcephalosporin C by cephalosporin C negative mutants of Acremonium chrysogenum. Agric Biol Chem 39: 1295–1302.
Gajewski W, Litwinska J, Paszewski A & Chojnacki T (1972) Isolation and characterization of lactose non-utilizing mutants in Aspergillus nidulans. Mol Gen Genet 116: 99–106.
Ganapathy ME, Brandsch M, Prasad PD, Ganapathy V & Leibach FH (1995) Differential recognition of β-lactam antibiotics by intestinal and renal peptide transporters, PEPT1 and PEPT2. J Biol Chem 270: 25672–25677.
Garrill A (1995) Transport. In: Gow NAR & Gadd GM (Eds) The Growing Fungus (pp 163–181). Chapman & Hall, London.
Gil-Espinosa S, Meesschaert BD & Martín JF (1993) Purification and characterization of a penicillin V acylase from Penicillium chrysogenum. Proc. VI Eur Congr Biotechnol, Abstr book, vol III, WE144, Florence.
Glover JR, Andrews DW & Rachubmski RA (1994) Saccharomyces cerevisiae peroxisomal thiolase is imported as a dimer. Proc Natl Acad Sci USA 91: 10541–10545.
Goldsmith J, Livoni JP, Norberg CL & Segel IH (1973) Regulation of nitrate uptake in Penicillium chrysogenum by ammonium ion. Plant Physiol 52: 362–367.
Gomord V & Faye L (1996) Signals and mechanisms involved in intracellular transport of secreted proteins in plants. Plant Physiol Biochem 34: 165–181.
Gooday GW (1995a) Cell walls. In: Gow NAR & Gadd GM (Eds) The Growing Fungus (pp 43–62). Chapman & Hall, London.
Gooday GW (1995b) Cell membrane. In: Gow NAR & Gadd GM (Eds) The Growing Fungus (pp 63–74). Chapman & Hall, London.
Gouka RJ, van Hartingsveldt W, Bovenberg RAL, van Zeijl CMJ, van den Hondel CAMJJ & van Gorcom RFM (1993) Development of a new transformant selection system for Penicillium chrysogenum: isolation and characterization of the P. chrysogenum acetyl-coezyme A synthetase gene (facA) and its use as a homologous selection marker. Appl Microbiol Biotechnol 38: 514–519.
Gow NAR & Gadd GM (1995) The Growing Fungus. Chapman & Hall, London.
Griffin DH (1994) Fungal Physiology, 2nd ed. Wiley-Liss, New York.
Griffith OW & Meister A (1981) 5-Oxo-L-prolinase (L-pyroglutamate hydrolase). Studies of the chemical mechanism. J Biol Chem 256: 9981–9985.
Grobler J, Bauer F, Subden RE & van Vuuren HJJ (1995) The mael gene of Schizosaccharomyces pombe encodes a permease for malate and other C-4 dicarboxylic acids. Yeast 11: 1485–1491.
Grondin K, Haimeur A, Mukhopadhyay R, Rosen BP & ouellette M (1997) Co-amplification of the γ-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J 16: 3057–3065.
Gutiérrez S, Díez B, Montenegro E & Martín JF (1991a) Characterization of the Cephalosporium acremonium pcbAB gene encoding δ-aminoadipyl-cysteinyl-valine synthetase, a large multidomain peptide synthetase: linkage to the pcbC gene as a cluster of early cephalosporin biosynthetic genes and evidence of multiple functional domains. J Bacteriol 173: 2354–2365.
Gutiérrez S, Díez B, Alvarez E, Barredo JL & Martín JF (1991b) Expression of the penDE gene of Penicillium chrysogenum encoding isopenicillin N acyltransferase in Cephalosporium acremonium: production of benzylpenicillin by the transformants. Mol Gen Genet 225: 56–64.
Gutiérrez S, Velasco J, Fernández FJ & Martín JF (1992) The cefG gene of Cephalosporium acremonium is linked to the cefEF gene and encodes a deacetylcephalosporin C acetyltransferase closely related to homoserine O-acetyltransferase. J Bacteriol 174: 3056–3064.
Gutiérrez S, Velasco J, Marcos AT, Fernández FJ, Fierro F, Barredo JL, Díez B & Martín JF (1997) Expression of the cefG gene is limiting for cephalosporin biosynthesis in Acremonium chrysogenum. Appl Microbiol Biotechnol 48: 606–614.
Gutiérrez S, Casqueiro J, Velasco J, Bañuelos O, Marcos AT & Martín JF (1998) Identification and elimination of bottlenecks in the penicillin and cephalosporin C biosynthesis. In: Martín JF & Gutiérrez S (Eds) Fungal Genetics — Fourth European Conference on Fungal Genetics (p 182). Universidad de León, León.
Haas H & Marzluf GA (1995) NRE, the major nitrogen regulatory protein of Penicillium chrysogenum, binds specifically to elements in the intergenic promoter regions of nitrate assimilation and penicillin biosynthetic gene clusters. Curr Genet 28: 177–183.
Haas H, Bauer B, Redl B, Stöfler G & Marzluf GA (1995) Molecular cloning and analysis of nre, the major nitrogen regulatory gene of Penicillium chrysogenum. Curr Genet 27: 150–158.
Hackette SL, Skye GE, Burton C & Segel IH (1970) Characterization of an ammonium transport system in filamentous fungi with methylammonium-14C as the substrate. J Biol Chem 245: 4241–4250.
Harter C & Wieland F (1996) The secretory pathway: mechanisms of protein sorting and transport. Biochim Biophys Acta 1286: 75–93.
Harvey WR & Nelson N (Eds) (1992) V-ATPases. J Exp Biol 172.
Hell R (1997) Molecular physiology of plant sulfur metabolism. Planta 202: 138–148.
Henriksen CM (1996) Metabolic characterization of Penicillium chrysogenum. PhD thesis. Technical University of Denmark, Lyngby
Henriksen CM, Christensen LH, Nielsen J & Villadsen J (1996) Growth energetics and metabolic fluxes in continuous cultures of Penicillium chrysogenum. J Biotechnol 45: 149–164.
Henriksen CM, Holm SS, Schipper D, Jørgensen HS, Nielsen J & Villadsen J (1997) Kinetic studies on the carboxylation of 6-aminopenicillanic acid to 8-hydroxypenillic acid. Process Biochem 32: 85–91.
Henriksen CM, Nielsen J & Villadsen J (1998) Cyclization of α-aminoadipic acid into the δ-lactam 6-oxopiperidine-2-carboxylic acid by Penicillium chrysogenum. J Antibiot 51: 99–106.
Hersbach GJM, van der Beek CP & van Dijck PWM (1984) The penicillins: properties, biosynthesis, and fermentation. In: Vandamme EJ (Ed) Biotechnology of Industrial Antibiotics (pp 45–140). Marcel Dekker, New York.
Hettema EH, van Roermund CWT, Distel B, van den Berg M, Vilela C, Rodrigues-Pousada C, Wanders RJA & Tabak HF (1996) The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acid into peroxisomes of Saccharomyces cerevisiae. EMBO J 15: 3813–3822.
Heupel R & Heldt HW (1994) Protein organization in the matrix of leaf peroxisomes. A multi-enzyme complex involved in photorespiratory metabolism. Eur J Biochem 220: 165–172.
Heupel R, Markgraf T, Robinson DG & Heldt HW (1991) Compartmentation studies on spinach leaf peroxisomes. Evidence for channeling of photorespiratory metabolites in peroxisomes devoid of intact boundary membrane. Plant Physiol 96: 971–979.
Higgins CF (1992) ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8: 67–113.
Hillenga DJ, Versantvoort HJM, Driessen AJM & Konings WN (1994) Structural and functional properties of plasma membranes from the filamentous fungus Penicillium chrysogenum. Eur J Biochem 224: 581–587.
Hillenga DJ, Versantvoort HJM, van der Molen S, Driessen AJM & Konings WN (1995) Penicillium chrysogenum takes up the penicillin G precursor phenylacetic acid by passive diffusion. Appl Environ Microbiol 61: 2589–2595.
Hillenga DJ, Versantvoort HJM, Driessen AJM & Konings WN (1996a) Sulfate transport in Penicillium chrysogenum plasma membranes. J Bacteriol 178: 3953–3956.
Hillenga DJ, Versantvoort HJM, Driessen AJM & Konings WN (1996b) Basic amino acid transport in plasma membrane vesicles of Penicillium chrysogenum. J Bacteriol 178: 3991–3995.
Hiruma R, Yamaguchi A & Sawai T (1984) The effect of lipopolysaccharide on lipid bilayer permeability of β-lactam antibiotics. FEES Lett 170: 268–272.
Hönlinger C & Kubicek CP (1989a) Regulation of δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine and isopenicillin N biosynthesis in Penicillium chrysogenum by the α-aminoadipate pool size. FEMS Microbiol Lett 65: 71–76.
Hönlinger C & Kubicek CP (1989b) Metabolism and compartmentation of α-aminoadipic acid in penicillin-producing strains of Penicillium chrysogenum. Biochim Biophys Acta 993: 204–211.
Horák J (1986) Amino acid transport in eucaryotic microorganisms. Biochim Biophys Acta 864: 223–256.
Horák J (1997) Yeast nutrient transporters. Biochim Biophys Acta 1331: 41–79.
Hunter DR & Segel IH (1971) Acidic and basic amino acid transport systems of Penicillium chrysogenum. Arch Biochem Biophys 144: 168–183.
Hunter DR & Segel IH (1973a) Control of the general amino acid permease of Penicillium chrysogenum by trans-inhibition and turnover. Arch Biochem Biophys 154: 387–399.
Hunter DR & Segel IH (1973b) Effect of weak acids on amino acid transport by Penicillium chrysogenum: Evidence for a proton or charge gradient as the driving force. J Bacteriol 113: 1184–1192.
Hunter DR & Segel IH (1985) Evidence for two distinct intracellular pools of inorganic sulfate in Penicillium notatum. J Bacteriol 162: 881–887.
Hussey C, Orsi BA, Scott J & Spencer B (1965) Mechanism of choline sulphate utilization in fungi. Nature 207: 632–634.
Isaac S, Gokhale AV & Wyatt AM (1986) Selectivity to K+ and Na+ of protoplast fractions isolated from different regions of Aspergillus nidulans hyphae. J Gen Microbiol 132: 1173–1179.
Iseki K, Naasani I, Kikuchi T, Sugawara M, Kobayashi M, Kohri N & Miyazaki K (1998) Purification and liposomal reconstitution of the oligopeptide transport activity in rat renal cortex using ceftibuten-affinity chromatography. Biochim Biophys Acta 1368: 329–337.
Ishikawa T (1992) The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci 17: 463–468.
Jacquemin-Faure I, Thomas D, Laporte J, Cibert C & Surdin-Kerjan Y (1994) The vacuolar compartment is required for sulfur amino acid homeostasis in Saccharomyces cerevisiae. Mol Gen Genet 244: 519–529.
Jaklitsch WM, Rohr M & Kubicek CP (1985) Glutamate pools and glutamate dehydrogenase regulation in relation to penicillin biosynthesis in strains of Penicillium chrysogenum. Exp. Mycol. 9: 310–317.
Jaklitsch WM, Hampel W, Röhr M, Kubicek CP & Gamerith G (1986) α-Aminoadipate pool concentration and penicillin biosynthesis in strains of Penicillium chrysogenum. Can J Microbiol 32: 473–480.
Jank B, Habermann B, Schweyen RJ & Link TA (1993) PMP47, a peroxisomal homologue of mitochondrial solute carrier proteins. Trends Biochem Sci 18: 427–128.
Jarai G & Marzluf GA (1991) Sulfate transport in Neurospora crassa: regulation, turnover, and cellular localization of the CYS-14 protein. Biochemistry 30: 4768–4773.
Jaspers C & Penninckx MJ (1984) Glutathione metabolism in the yeast Saccharomyces cerevisiae. Evidence that γ-glutamyl transpeptidase is a vacuolar enzyme. Biochimie 66: 71–74.
Jennings DH (1995) The Physiology of Fungal Nutrition. Cambridge University Press, Cambridge.
Jensen SE, Leskiw BK, Vining LC, Aharonowitz Y & Westlake DWS (1986) Purification of isopenicillin N synthetase from Streptomyces clavuligerus. Can J Microbiol 32: 953–958.
Jensen SE, Westlake DWS & Wolfe S (1988) Production of the penicillin precursor δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine (ACV) by cell-free extracts from Streptomyces clavuligerus. FEMS Microbiol Lett 49: 213–218.
Jensen SE, Alexander DC, Paradkar AS & Aidoo KW (1993) Extending the β-lactam biosynthetic gene cluster in Streptomyces clavuligerus. In: Baltz RH, Hegeman GD & Skatrud PL (Eds) Industrial Microorganisms: Basic and Applied Molecular Genetics (pp 257–265). Amer Soc Microbiol, Washington, DC.
Jones EW, Webb GC & Hiller MA (1997) Biogenesis and function of the yeast vacuole. In: Pringle JR, Broach JR & Jones EW (Eds) Molecular Biology of the Yeast Saccharomyces cerevisiae, vol III: Cell Cycle and Cell Biology (pp 363–169). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Jørgensen H (1993) Metabolic fluxes in Penicillium chrysogenum. PhD Thesis. Technical University of Denmark, Lyngby.
Jørgensen H, Nielsen J, Villadsen J & Møllgaard H (1995a) Metabolic flux distributions in Penicillium chrysogenum during fed-batch cultivations. Biotechnol Bioeng 46: 117–131.
Jørgensen H, Nielsen J, Villadsen J & Møllgaard H (1995b) Analysis of penicillin V biosynthesis during fed-batch cultivations with a high-yielding strain of Penicillium chrysogenum. Appl Microbiol Biotechnol 43: 123–130.
Kadima TA, Jensen SE & Pickard MA (1995) Production of δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine by entrapped ACV-synthetase from Streptomyces clavuligerus. J Industr Microbiol 14: 35–40.
Kakinuma Y, Ohsumi Y & Anraku Y (1981) Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem 256: 10859–10863.
Kallow W, von Döhren H & Kleinkauf H (1998) Penicillin biosynthesis: energy requirement for tripeptide precursor formation by δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine synthetase from Acremonium chrysogenum. Biochemistry 37: 5947–5952.
Kamijo K, Taketani S, Yokota S, Osumi T & Hashimoto (1990) The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J Biol Chem 265: 4534–1540.
Kennedy J & Turner G (1996) δ-(L-α-Aminoadipyl)-L-cysteinyl-D-valine synthetase is a rate limiting enzyme for penicillin production in Aspergillus nidulans. Mol Gen Genet 253: 189–197.
Ketter JS & Marzluf GA (1988) Molecular cloning and analysis of the regulation of cys-14 +, a structural gene of the sulfur regulatory circuit of Neurospora crassa. Mol Cell Biol 8: 1504–1508.
Ketter JS, Jarai G, Fu Y-H & Marzluf GA (1991) Nucleotide sequence, messenger RNA stability, and DNA recognition elements of cys-14, the structural gene for sulfate permease II in Neurospora crassa. Biochemistry 30: 1780–1787.
Kimura T, Yoshikawa M, Yasuhara M & Sezaki H (1980) The use of liposomes as a model of drug absorption: β-lactam antibiotics. J Pharm Pharmacol 32: 394–398.
Kionka C & Kunau W-H (1985) Inducible β-oxidation pathway in Neurospora crassa. J Bacteriol 161: 153–157.
Kirschbaum J (1986) Penicillin G, potassium. In: Florey K (Ed) Analytical Profiles of Drug Substances, Vol 15 (pp 427–509). Academic Press, New York.
Kitamoto K, Yoshizawa K, Ohsumi Y & Anraku Y (1988) Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J Bacteriol 170: 2683–2686.
Kleinkauf H & von Döhren H (Eds) (1991) 50 Years of Penicillin Application — History and Trends. Public, Czech Republic.
Kleinkauf H & von Döhren H (1996) A nonribosomal system of peptide biosynthesis. Eur J Biochem 236: 335–351.
Klionsky DJ (1997) Protein transport from the cytoplasm into the vacuole. Membrane Biol. 157: 105–115.
Klionsky DJ (1998) Nonclassical protein sorting to the yeast vacuole. J Biol Chem 273: 10807–10810.
Klionsky DJ, Herman PK & Emr SD (1990) The fungal vacuole: composition, function, and biogenesis. Microbiol Rev 54: 266–292.
Koehler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty LK & Pechere J-C (1997) Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol 23: 345–354.
Kogekar RG & Deshpande VN (1982) Biosynthesis of penicillin in vitro: purification and properties of ‘phenyl/phenoxyacetic acid activating enzyme’. Indian J Biochem Biophys 19: 257–261.
Kolling R & Hollenberg CP (1994) The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. EMBO J 13: 3261–3271.
Kovacevic S & Miller JR (1991) Cloning and sequencing of the β-lactam hydroxylase gene (cefF) from Streptomyces clavuligerus: gene duplication may have led to separate hydroxylase and expandase activities in the actinomycetes. J Bacteriol 173: 398–400.
Kovacevic S, Weigel BJ, Tobin MB, Ingolia TD & Miller JR (1989) Cloning, characterization, and expression in Escherichia coli of the Streptomyces clavuligerus gene encoding deacetoxy-cephalosporin C synthetase. J Bacteriol 171: 754–760.
Kovacevic S, Tobin MB & Miller JR (1990) The β-lactam biosynthesis genes for isopenicillin N epimerase and deacetoxy-cephalosporin C synthetase are expressed from a single transcript in Streptomyces clavuligerus. J Bacteriol 172: 3952–3958.
Krämer R (1996) Analysis and modeling of substrate uptake and product release by prokaryotic and eukaryotic cells. Adv Biochem Engin/Biotechnol 54: 31–74.
Kramer W, Girbig F, Gutjahr U, Kowalewski S, Adam F & Schiebler W (1992) Intestinal absorption of β-lactam antibiotics and oligopeptides. Functional and stereospecific reconstitution of the oligopeptide transport system from rabbit small intestine. Eur J Biochem 204: 923–930.
Kruckeberg AL (1996) The hexose transporter family of Saccharomyces cerevisiae. Arch Microbiol 166: 283–292.
Kuan J & Saier MH Jr (1993) The mitochondrial carrier family of transport proteins: structural, functional, and evolutionary relationships. Crit Rev Biochem Mol Biol 28: 209–233.
Kubicek CP, Hönlinger C, Jaklitsch WM, Affenzeller K, Mach R, Gerngross TU & Lu Y (1990) Regulation of lysine biosynthesis in the fungus Penicillium chrysogenum. In: Lubec G & Rozenthal GA (Eds) Amino Acids: Chemistry, Biology and Medicine. ESCOM Science Publishers, Leiden.
Kubicek-Pranz EM & Kubicek CP (1991) Production and biosynthesis of amino acids by fungi. In: Arora DK, Elander RP & Mukerji KG (Eds) Handbook of Applied Mycology, Vol 4: Fungal Biotechnology (pp 313–356). Marcel Dekker, New York.
Kuhn PJ, Trinci APJ, Jung MJ, Goosey MW & Copping LG (Eds) (1989) The Biochemistry of Cell Walls and Membranes in Fungi. Springer Verlag, Berlin.
Kühn MJ, Schekman R & Ljungdahl PO (1996) Amino acid permeases require COPII components and the ER resident membrane protein Shr3p for packaging into transport vesicles in vitro. J Cell Biol 135: 585–595.
Kupka J, Shen Y-Q, Wolfe S & Demain AL (1983) Studies on the ring cyclization and ring-expansion enzymes of β-lactam biosynthesis in Cephalosporium acremonium. Can J Microbiol 29: 488–496.
Kuryłowicz W, Kurzatkowski W & Kurzatkowski J (1987) Biosynthesis of benzylpenicillin by Penicillium chrysogenum and its Golgi apparatus. Arch Immunol Ther Exp 35: 699–724.
Kurzatkowski W & Kuryłowicz W (1991) Penicillium chrysogenum in course of biosynthesis of penicillin G. In: Kleinkauf H & von Döhren H (Eds) 50 Years of Penicillin Application — History and Trends (pp 237–243). Public, Czech republic.
Kurzatkowski W, Palissa H, Van Liempt H, von Döhren H, Kleinkauf H, Wolf WP & Kuryłowicz W (1991) Localization of isopenicillin N synthase in Penicillium chrysogenum PQ-96. Appl Microbiol Biotechnol 35: 517–520.
Láiz L, Liras P, Castro JM & Martín JF (1990) Purification and characterization of the isopenicillin N epimerase from Nocardia lactamdurans. J Gen Microbiol 136: 663–671.
Lara F, Mateos RC, Vázquez G & Sánchez S (1982) Induction of penicillin biosynthesis by L-glutamate in Penicillium chrysogenum. Biochem Biophys Res Commun 105: 172–178.
Lazarow PB & Kunau WH (1997) Peroxisomes. In: Pringle JR, Broach JR & Jones EW (Eds) Molecular Biology of the Yeast Saccharomyces cerevisiae, Vol III: Cell Cycle and Cell Biology (pp 547–606). Cold Spring Harbor Laboratory Press, Cold Spring Harbor NY.
Leal-Morales CA, Bracker CE & Bartnicki-Garcia S (1994) Distribution of chitin synthetase and various membrane marker enzymes in chitosomes and other organelles of the slime mutant of Neurospora crassa. Exp Mycol 18: 168–179.
Lendenfeld T, Ghali D, Wolchek M, Kubicek-Pranz EM & Kubicek CP (1993) Subcellular compartmentation of penicillin biosynthesis in Penicillium chrysogenum — the amino acids are derived from the vacuole. J Biol Chem 268: 665–671.
Leskiw BK, Aharonowitz Y, Mevarech M, Wolfe S, Vining LC, Westlake DWS & Jensen SE (1988) Cloning and nucleotide sequence determination of the isopenicillin N synthetase gene from Streptomyces clavuligerus. Gene 62: 187–196.
Li Z-S, Zhao Y & Rea PA (1995) Magnesium adenosine 5′-triphosphate-energized transport of glutathione-S-conjugates by plant vacuolar membrane vesicles. Plant Physiol 107: 1257–1268.
Li Z-S, Szcypka M, Lu Y-P, Thiele DJ & Rea PA (1996) The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem 271: 6509–6517.
Li Z-S, Lu Y-P, Zhen R-G, Szczypka M, Thiele DJ & Rea PA (1997) A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathione)cadmium. Proc Natl Acad Sci USA 94: 42–7.
Liersch M, Nüesch J & Treichler J (1976) Final steps in the biosynthesis of cephalosporin C. In: MacDonald KD (Ed) Second International Symposium on the Genetics of Industrial Microorganisms (pp 179–195). Acad Press, London.
Liu Y, Peter D, Merickel A, Krantz D, Finn JP & Edwards RH (1995) A molecular analysis of vesicular transport. Behav Brain Res 73: 51–58.
Logan H, Basset M, Véry A-A & Sentenac H (1997) Plasma membrane transport systems in higher plasnts: from black boxes to molecular physiology. Physiol Plantarum 100: 1–15.
Lombard-Platet G, Savary S, Sarde CO, Mandel JL & Chimini G (1996) A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc Natl Acad Sci USA 93: 1265–1269.
López-Nieto MJ, Ramos FR, Luengo JM & Martín JF (1985) Characterization of the biosynthesis in vivo of α-aminoadipyl-cysteinyl-valine in Penicillium chrysogenum. Appl Microbiol Biotechnol 22: 343–351.
Lösel DM (1989) Lipids in the structure and function of fungal membranes. In: Kuhn PJ, Trinci APJ, Jung MJ, Goosey MW & Copping LG (Eds) The Biochemistry of Cell Walls and Membranes in Fungi (pp 119–133). Springer Verlag, Berlin
Lu Y, Mach RL, Affenzeller K & Kubicek CP (1992) Regulation of α-aminoadipate reductase from Penicilliumchrysogenum in relation to the flux from α-aminoadipate into penicillin biosynthesis. Can J Microbiol 38: 758–763.
Lu Y-P, Li Z-S, Drozdowicz YM, Hörstensteiner S, Martinoia E & Rea PA (1998) AtMRP2, and Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell 10: 267–282.
Lübbe C, Wolfe S & Demain AL (1986) Isopenicillin N epimerase activity in a high cephalosporin-producing strain of Cephalosporium acremonium. Appl Microbiol Biotechnol 23: 367–368.
Luengo JM (1995) Enzymatic synthesis of hydrophobic penicillins. J Antibiot 48: 1195–1212.
Luengo JM, Domingez JM, Cantoral JM & Martín JF (1986) Formation of bulges associated with penicillin productionin high-producing strains of Penicillium chrysogenum. Curr Microbiol 13: 203–207.
Ma D, Cook DN, Hearst JE & Nikaido H (1994) Efflux pumps and drug resistance in Gram-negative bacteria. Trends Microbiol 2: 489–493.
MacCabe AP, van Liempt H, Palissa H, Unkles SE, Riach MBR, Pfeifer E, von Döhren H & Kinghorn JR (1991a) δ-(L-α-Aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. Molecular characterization of the acvA gene encoding the first enzyme of the penicilline biosynthetic pathway. J Biol Chem 266: 12646–12654.
MacCabe AP, Riach MBR & Kinghorn JR (1991b) Identification and expression of the ACV synthetase gene. J Biotechnol 17: 91–97
Madi L, McBride SA, Bailey LA & Ebbole DJ (1997) rco-3, a gene involved in glucose transport and conidiation in Neurospora crassa. Genetics 146: 499–508.
Maeda K, Luengo JM, Ferrero O, Wolfe S, Lebedev MY, Fang A & Demain AL (1995) The substrate specificity of deacetoxycephalosporin C synthase (’expandase’) of Streptomyces clavuligerus is extremely narrow. Enz Microbial Technol 17: 231–234.
Marahiel MA, Stachelhaus T & Mootz HD (1997) Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem Rev 97: 2651–2673.
Marger MD & Saier MH Jr (1993) A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci 18: 13–20.
Mark CG & Romano AH (1971) Properties of the hexose transport systems of Aspergilllus nidulans. Biochim BiophysActa 249: 216–226.
Markham P (1995) Organelles of filamentous fungi. In: Gow NAR & Gadd GM (Eds) The Growing Fungus (pp 75–98). Chapman & Hall, London.
Martín JF & Aharonowitz Y (1983) Regulation of biosynthesis of β-lactam antibiotics. In: Demain AL & Solomon NA (Eds) Antibiotics Containing the β-Lactam Structure (pp 229–254). Springer-Verlag, Berlin.
Martín JF, Luengo JM, Revilla G & Villnueva JR (1979) Biochemical genetics of the β-lactam antibiotic biosynthesis. In: Sebek OK & Laskin AI (Eds) Genetics of Industrial Microorganisms (pp 83–89). Am Soc Microbiol, Washington, DC.
Martín JF & Gutiérrez S (1995) Genes for β-lactam antibiotic biosynthesis. Antonie van Leeuwenhoek 67: 181–200.
Martín JF & Liras P (1989a) Enzymes involved in penicillin, cephalosporin and cephamycin biosynthesis. Adv Biochem Eng/Biotechnol 39: 153–187.
Martín JF, Ingolia TD & Queener SW (1991) Molecular genetics of penicillin and cephalosporin antibiotic biosynthesis. In: Leong SA & Berka RM (Eds) Molecular Industrial Mycology: Systems and Applications for Filamentous Fungi (pp 149–196). Marcel Dekker, New York.
Martín JF, Gutiérrez S & Demain AL (1997) β-Lactams. In: Anke T (Ed) Fungal Biotechnology. Antibiotics (pp 91–127). Chapman & Hall, Weinheim.
Martínez-Blanco H, Reglero A, Ferrero MA, Fernández-Cañón JM & Luengo JM (1989) III. Repression of phenyl-acetic acid transport system in Penicillium chrysogenum Wis 54–1255 by free amino acids and ammmonium salts. J Antibiot 42: 1416–1423.
Martínez-Blanco H, Reglero A, Rodríguez-Aparacio LB & Luengo JM (1990) Purification and biochemical characterization of phenylacetyl-CoA ligase from Pseudomonas putida. J Biol Chem 265: 7084–7090.
Martínez-Blanco H, Reglero A, Fernández-Valverde M, Ferrero MA, Moreno MA, Peñalva MA & Luengo JM (1992) Isolation and characterization of the acetyl-CoA synthetase from Penicillium chrysogenum — involvement of this enzyme in the biosynthesis of penicillins. J Biol Chem 267: 5474–5481.
Martínez-Blanco H, Orejas M, Reglero A, Luengo JM & Peñalva MA (1993) Characterisation of the gene encoding acetyl-CoA synthetase in Penicillium chrysogenum: conservation of intron position in plectomycetes. Gene 130: 265–270.
Martinoia E, Grill E, Tommasini R, Kreuz K & Amrhein N (1993) ATP-dependent glutathione S-conjugate ‘export’ pump in the vacuolar membrane of plants. Nature 364: 247–249.
Marzluf GA (1970a) Genetic and metabolic controls for sulfate metabolism in Neurospora crassa: isolation and study of chromate-resistant and sulfate transport negative mutants. J Bacteriol 102: 716–721.
Marzluf GA (1970b) Genetic and biochemical studies of distinct sulfate permease species in different developmental stages of Neurospora crassa. Arch Biochem Biophys 138: 254–263.
Marzluf GA (1997a) Molecular genetics of sulfur assimilation in filamentous fungi and yeast. Annu Rev Microbiol 51: 73–96.
Marzluf GA (1997b) Genetic regulation of nitrogen metabolism in the fungi. Microbiol Molec Biol Rev 61: 17–32.
Mathison L, Soliday C, Stepan T, Aldrich T & Rambosek J (1993) Cloning, characterization, and use in strain improvement of the Cephalosporium acremonium gene cefG encoding acetyl transferase. Curr Genet 23: 33–41.
Matsudo A, Sugiura H, Matsuyama K, Matsumoto H, Ichikawa S & Komatsu K-I (1992) Cloning and disruption of the cefG gene encoding acetyl coenzyme A: deacetylcephalosporin O-acetyltransferase from Acremonium chrysogenum. Biochem Biophys Res Commun 186: 40–46.
Maxwell DP, Maxwell MD, Hänssler G, Armentrout VN, Murray GM & Hoch HC (1975) Microbodies and glyoxylate-cycle enzyme activities in filamentous fungi. Planta 124: 109–123.
McCammon MX, McNew JA, Willy PJ & Goodman JM (1994) An internal region of the peroxisomal membrane protein PMP47 is essential for sorting to peroxisomes. J Cell Biol 124: 915–925.
McNew JA & Goodman JM (1994) An oligomeric protein is imported in peroxisomes in vivo. J Cell Biol 127: 1245–1257.
McNew JA & Goodman JM (1996) The targeting and assembly of peroxisomal proteins: some old rules do not apply. Trends Biochem Sci 21: 54–58.
Meesschaert B, Gil-Espinosa S & Martín JF (1991) Extraction, purification and partial characterization of a penicillinV acylase from Penicillium chrysogenum. Med Fac Landbouww Rijksuniv Gent 56: 1781–1783.
Mehdi K & Penninckx MJ (1997) An important role for glutathione and γ-glutamyltranspeptidase in the supply of growth requirements during nitrogen starvation of the yeast Saccharomyces cerevisiae. Microbiol 143: 1885–1889.
Meier PJ (1995) Molecular mechanisms of hepatic bile salt transport from sinusoidal blood into bile. Amer J Physiol 269: G801–812
Meister A (1988) Glutathione metabolism and its. selective modification. J Biol Chem 263: 17205–17208.
Meister A & Anderson ME (1983) Glutathione. Annu Rev Biochem 52: 711–760.
Méndez C & Salas JA (1998) ABC transporters in antibiotic-producing actinomycetes. FEMS Microbiol Lett 158: 1–8.
Miñambres B, Martínez-Blanco H, Olivera ER, García B, Díez B, Barredo JL, Moreno MA, Schleissner C, Salto F& Luengo JM (1996) Molecular cloning and expression in different microbes of the DNA encoding Pseudomonas putida U phenylacetyl-CoA ligase. Use of this gene to improve the rate of benzylpenicillin biosynthesis in Penicillium chrysogenum. J Biol Chem 271: 33531–33538.
Montenegro E, Barredo JL, Gutiérrez S, Díez B, Alvarez E & JF Martín (1990) Cloning, characterization of the acyl-CoA:6-amino penicillanic acid acyltransferase gene of Aspergillus nidulans and linkage to the isopenicillin N synthase gene. Mol Gen Genet 221: 322–330.
Moriyama Y, Yamamoto A, Yamada H, Tashiro Y & Futai M (1996) Role of endocrine cell microvesicles in inter-cellular chemical transduction. Biol Chem Hoppe-Seyler 377: 155–165.
Mosser J, Douar A-M, Sarde C-O, Kioschis P, Feil R, Moser H, Poustka A-M, Mandel J-L & Aubourg P (1993) Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361: 726–730.
Müller W (1991) Localization of penicillin biosynthesis in Penicillium chrysogenum. PhD thesis. University of Utrecht, Utrecht.
Müller WH, van der Krift TP, Krouwer AJJ, Wösten HAB, van der Voort LHM, Smaal EB & Verkleij AJ (1991) Localization of the pathway of the penicillin biosynthesis in Penicillium chrysogenum. EMBO J 10: 489–495.
Müller WH, Bovenberg RAL, Groothuis MH, Kattevilder F, Smaal EB, van der Voort LHM & Verkleij AJ (1992) Involvement of microbodies in penicillin biosynthesis. Biochim Biophys Acta 1116: 210–213.
Müller WH, Essers J, Humbel BM & Verkleij AJ (1995) Enrichment of Penicillium chrysogenum microbodies byisopycnic centrifugation in nycodenz as visualized with immuno-electron microscopy. Biochim Biophys Acta 1245: 215–220.
Naasani I, Kikuchi T, Sugawara M, Kobayashi M, Iseki K & Miyazaki K (1996) Solubilization and reconstitution characteristics of the carrier protein(s) responsible for the transport of ceftibuten, a substrate for the oligopeptide transporters, in rat renal brush-border membrane. Biochim Biophys Acta 1283: 185–191.
Nakaune R, Adachi K, Nawata O, Tomiyama M, Akutsu K & Hibi T (1998) A novel ATP-binding cassette transporter involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Appl Eniron Microbiol 64: 3983–3988.
Nelissen B, De Wachter R & Goffeau A (1997) Classification of all putative permeases and other membrane plurispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol Rev 21: 113–134.
Nelson N (1992) Organellar proton-ATPases. Curr Opin Cell Biol 4: 654–660.
Newbert RW, Barton B, Greaves P, Harper J & Turner G (1997) Analysis of a commercially improved Penicillium chrysogenum strain series: involvement of recombigenic regions in amplification and deletion of the penicillin biosynthesis gene cluster. J Industr Microbiol Biotechnol 19: 18–27.
Nicolay K, Veenhuis M, Douma AC & Harder W (1987) A 31P NMR study of the internal pH of yeast peroxisomes. Arch Microbiol 147: 37–41.
Nielsen J (1995) Physiological engineering aspects of Penicillium chrysogenum. Technical University of Denmark, Lyngby.
Nielsen J (1998) The role of metabolic engineering in the production of secondary metabolites. Curr Opin Microbiol 1: 330–336.
Nielsen J & Jørgensen HS (1995) Metabolic control analysis of the penicillin biosynthetic pathway in a high-yielding strain of Penicillium chrysogenum. Biotechnol Prog 11: 299–305.
Nielsen J & Krabben P (1995) Hyphal growth and fragmentation of Penicillium chrysogenum in submerged cultures. Biotechnol Bioeng 46: 588–598.
Nikaido H (1994) Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264: 382–388.
Nikaido H, Basina M, Nguen V & Rosenberg EY (1998) Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J Bacteriol 180: 4686–4692.
Noctor G & Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279.
Nüesch J, Hinnen A, Liersch M & Treichler HJ (1976) A biochemical and genetic approach to the biosynthesis of cephalosporin C. In: MacDonald KD (Ed) Second International Symposium on the Genetics of Industrial Microorganisms (pp 451–472). Academic Press, London
Nüesch J, Heim J & Treichler H-J (1987) The biosynthesis of sulfur-containing β-lactam antibiotics. Annu Rev Microbiol 41: 51–75.
Osmundsen H, Bremer J & Pedersen JI (1991) Metabolic aspects of peroxisomal β-oxidation. Biochim Biophys Acta 1085: 141–158.
Packer HL, Keshavarz-Moore E, Lilly MD & Thomas CR (1992) Estimation of cell volume and biomass of Penicillium chrysogenum using image analysis. Biotechnol Bioeng 39: 384–391.
Pang C-P, Chakravarti B, Adlington RM, Ting H-H, White RL, Jayatilake GS, Baldwin JE & Abraham EP (1984) Purification of isopenicillin N synthetase. Biochem J 222: 789–795.
Pao SS, Paulsen IT & Saier MH Jr (1998) Major facilitator superfamily. Microbiol Mol Biol Rev 62: 1–34.
Paul GC & Thomas CR (1996) A structured model for hyphal differentiation and penicillin production using Penicillium chrysogenum. Biotechnol Bioeng 51: 558–572.
Paul GC, Kent CA & Thomas CR (1994) Hyphal vacuolation and fragmentation in Penicillium chrysogenum. Biotechnol Bioeng 44: 655–660.
Paulsen IT, Sliwinski MK, Nelissen B, Goffeau A & Saier MH Jr (1998) Unified inventory of established and putative transporters encoded within the complete genome of Saccharomyces cerevisiae. FEES Lett 430: 116–125.
Payne GM & Payne JW (1984) γ-Glutamyltransferase is not involved in the bulk uptake of amino acids, peptides or γ-glutamylamino acids in yeast (Saccharomyces cerevisiae). Biochem J 218: 147–155.
Payne JW & Smith MW (1994) Peptide transport by microorganisms. Adv Microbial Physiol 36: 1–80.
Pelham HRB (1996) The dynamic organisation of the secretory pathway. Cell Struct Function 21: 413–419.
Peñalva MA, Espeso E, Orejas M & Gomez-Pardo E (1991) Evolution and control of gene expression of the Aspergillus nidulans isopenicillin N synthase gene. In: Kleinkauf H & von Döhren H (Eds) 50 Years of Penicillin Application — History and Trends (pp 224–230). Public, Czech Republic.
Peñalva MA, Pérez-Esteban B, Gomez-Pardo E, Orejas M & Espeso E (1992) Penicillin production by Aspergillus nidulans: studies on the regulation of expression of the IPNS gene. In: Stahl U & Tudzynski P (Eds) Molecular Biology of Filamentous Fungi (pp 217–227). VCH, Weinheim.
Penninckx MJ & Elskens MT (1993) Metabolism and functions of glutathione in micro-organisms. Adv Microbial Physiol 34: 239–301.
Pierre MV St, Ruetz S, Epstein LF, Gros P & Arias IM (1994) ATP-dependent transport of organic anions in secretory vesicles of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 91: 9476–9479.
Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J-I, Li X-Z & Nishino T (1996) Overexpression of the mexC-mex-oprJ efflux operon in nfxB multidrug resistant strains of Pseudomonas aeruginosa. Mol Microbiol 21: 713–724.
Pringle JR, Broach JR & Jones EW (Eds) (1997) Molecular Biology of the Yeast Saccharomyces cerevisiae, Vol III: Cell Cycle and Cell Biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Pusztahelyi T, Pócsi I, Kozma J & Szentirmai A (1997a) Aging of Penicillium chrysogenum cultures under carbon starvation: I: morphological changes and secondary metabolite production. Biotechnol Appl Biochem 25: 81–86.
Pusztahelyi T, Pócsi I & Szentirmai A (1997b) Aging of Penicillium chrysogenum cultures under carbon starvation: II: protease and N-acetyl-β-D-hexosaminidase production. Biotechnol Appl Biochem 25: 87–93.
Rachubinski RA & Subramani S (1995) How proteins penetrate peroxisomes. Cell 83: 525–528.
Ramón D, Carramolino L, Patiño C, Sánchez F & Peñalva MA (1987) Cloning and characterisation of the isopenicillin N synthetase gene mediating the formation of the β-lactam ring in Aspergillus nidulans. Gene 57: 171–181.
Ramos FR, López-Nieto MJ & Martín JF (1985) Isopenicillin N synthetase of Penicillium chrysogenum, an enzyme that converts δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother 27: 380–387.
Rao R & Slayman CW (1996) Plasma-membrane and related ATPases. In: Brambl R & Marzluf GA (Eds) The Mycota III (pp 29–56). Springer-Verlag, Berlin.
Rea PA, Li ZS, Lu YP, Drozdowicz YM & Martinoia E (1998) From vacuolar GS-X pumps to multispecific ABC transporters. Annu Rev Plant Physiol Plant Mol Biol 49: 727–760.
Regalado CM, Sleeman BD & Ritz K (1997) Aggregation and collapse of fungal wall vesicles in hyphal tips: a model for the origin of Spitzenkörper. Phil Trans R Soc Lond B 352: 1963–1974.
Reoyo E, Espeso EA, Peñalva MA & Suárez T (1998) The Aspergillus nidulans gene pmaA encodes a polypeptidehomologue of plasma membrane H+-ATPases. In: Martín JF & Gutiérrez S (Eds) Fungal Genetics — Fourth European Conference on Fungal Genetics (p 54). Universidad de León, León.
Reumann S, Heupel R & Heldt HW (1994) Compartmentation studies on spinach leaf peroxisomes. II. Evidence for the transfer of reductant from the cytosol to the peroxisomal compartment via a malate shuttle. Planta 193: 167–173.
Reumann S, Maier E, Benz & Heldt HW (1995) The membrane of leaf peroxisomes contains a porin-like channel. J Biol Chem 270: 17559–17565.
Revilla G, Ramos FR, López-Nieto MJ, Alvarez E & Martín JF (1986) Glucose represses formation of δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine and isopenicillin N synthase but not penicillin acyltransferase in Penicillium chrysogenum. J Bacteriol 168: 947–952.
Roach PL, Clifton IJ, Fülöp V, Harlos K, Barton GJ, Hajdu J, Andersson I, Schofield CJ & Baldwin JE (1995) Crystal structure of isopenicillin N synthase is the first from a new structural family of enzymes. Nature 375: 700–704.
Roach PL, Clifton IJ, Hensgens CMH, Shibata N, Schofield CJ, Hajdu J & Baldwin JE (1997) Structure of isopenicillin N synthase complexed with substrate and the mechanism of penicillin formation. Nature 387: 827–830.
Rodríguez M, Barredo JL & Diéz B (1998) Oxidative metabolism of phenylacetic acid in Penicillium chrysogenum. In: Martín JF & Gutiérrez S (Eds) Fungal Genetics — Fourth European Conference on Fungal Genetics (p 189). Universidad de León, León.
Roncal T, Ugalde UO & Irastorza A (1993) Calcium-induced conidiation in Penicillium cyclopium: Calcium triggers cytosolic alkanalization at the hyphal tip. J Bacteriol 175: 879–886.
Roos W (1989) Kinetic properties, nutrient-dependent regulation and energy coupling of amino-acid transport systems in Penicillium cyclopium. Biochim Biophys Acta 978: 119–133.
Roos W, Schulze R & Steighardt J (1997) Dynamic compartmentation of vacuolar amino acids in Penicillium cyclopium. J Biol Chem 272: 15849–15855.
Ruiz-Herrera J (1992) Fungal Cell Wall: Structure, Synthesis, and Assembly. CRC Press, Boca Raton, FL.
Saier MH Jr (1998) Molecular phylogeny as a basis for the classification of transport proteins from bacteria, archea and eukarya. Adv Microbial Physiol 40: 81–136.
Sakai Y, Saiganji A, Yurimoto H, Takabe K, Saiki H & Kato N (1996) The absence of Pmp47, a putative yeast peroxisomal transporter, causes a defect in transport and folding of a specific matrix enzyme. J Cell Biol 134: 37–51.
Samson SM, Belagaje R, Blankenship DT, Chapman JL, Perry D, Skatrud PL, VanFrank RM, Abraham EP, Baldwin JE, Queener SW & Ingolia TD (1985) Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthase gene from Cephalosporium acremonium. Nature 318: 191–194.
Samson SM, Dotzlaf JE, Slisz ML, Becker GW, van Frank RM, Veal LE, Yeh W-K, Miller JR, Queener SW & Ingolia TD (1987) Cloning and expression of the fungal expandase/hydroxylase gene involved in cephalosporin biosynthesis. Bio/Technology 5: 1207–1216.
Sánchez S, Flores ME & Demain AL (1988) Nitrogen regulation of penicillin and cephalosporin fermentations. In: Sanchez-Esquivel S (Ed) Nitrogen Source Control of Microbial Processes (pp 121–136). CRC Press, Boca Raton, FL.
Sanders D & Slayman CL (1982) Control of intracellular pH. Predominant role of oxidative metabolism on proton transport in the eukaryotic microorganism Neurospora. J Gen Physiol 80: 377–402.
Sato T, Ohsumi Y & Anraku Y (1984a) Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. J Biol Chem 259: 11505–11508.
Sato T, Ohsumi Y & Anraku Y (1984b) An arginine/histidine exchange transport system in vacuolar-membrane vesicles of Saccharomyces cerevisiae. J Biol Chem 259: 11509–11511.
Scarborough GA (1969) Sugar transport in Neurospora crassa. J Biol Chem 245: 1694–1698.
Scarborough GA (1970) Sugar transport in Neurospora crassa. II. A second glucose transport system. J Biol Chem 245: 3985–3987.
Scarborough GA (1996) The Neurospora plasma membrane proton pump. In: Konings WN, Kaback HR & Lolkema JS (Eds) Handbook of Biological Physics (pp 75–92). Elsevier, Cambridge.
Schekman R (1992) Genetic and biochemical analysis of vesicular traffic in yeast. Curr Opin Cell Biol 4: 587–592.
Schilling B & Lerch K (1995) Cloning, sequencing and heterologous expression of the monoamine oxidase gene from Aspergillus niger. Mol Gen Genet 247: 430–438.
Schleissner C, Olivera ER, Fernández-Valverde M & Luengo JM (1994) Aerobic catabolism of phenylacetic acid in Pseudomonas putida U: biochemical characterization of a specific phenylacetic acid transport system and formal demonstration that phenylacetyl-coenzyme A is a catabolic intermediate. J Bacteriol 176: 7667–7676.
Schneider RP & Wiley WR (1971b) Regulation of sugar transport in Neurospora crassa. J Bacteriol 106: 487–492.
Schneider RP & Wiley WR (1971a) Kinetic characterization of the two glucose transport systems in Neurosporacrassa. J Bacteriol 106: 479–486.
Schügerl K (Ed) (1998) Relationship between Morphology and Process Performances. Adv Biochem Engin/Biotechnol Vol 60. Springer-Verlag, Berlin.
Schwartz R, Lucas MT, Escalante L, Vázquez G & Sánchez S (1988) Glutathione formation in Penicillium chrysogenum: stimulatory effect of ammonium. J Gen Microbiol 134: 1117–1121.
Schwecke T, Aharonowitz Y, Palissa H, van Döhren H, Kleinkauf H & van Liempt H (1992) Enzymatic characterization of the multifunctional enzyme δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine synthetase from Streptomyces clavuligerus. Eur J Biochem 205: 687–694.
Segel IH & Johnson MJ (1960) Accumulation of intracellular inorganic sulfate by Penicillium chrysogenum. JBacteriol 81: 91–98.
Sentandreu R, Mormeno S & Ruiz-Herrera J (1994) Biogenesis of the fungal cell wall. In: Wessels JGH & Meinhhardt F (Eds) The Mycota I (pp 111–124). Springer-Verlag, Berlin, Heidelberg.
Shani N & Valle D (1998) Peroxisomal ABC transporters. Methods Enzymol 292: 753–776.
Shani N, Watkins PA & Valle D (1995) PXA1, a possible Saccharomyces cerevisiae ortholog of the human adrenoleukodystrophy gene. Proc Natl Acad Sci USA 92: 6012–6016.
Shani N, Sapag A & Valle D (1996) Characterization and analysis of conserved motifs in a peroxisomal ATP-binding cassette transporter. J Biol Chem 271: 8725–8730.
Sieńko M, Topczewski J & Paszewski A (1998) Structure and regulation of cysD, the homocysteine sythase gene of Aspergillus nidulans. Curr Genet 33: 136–144.
Sietsma JH & Wessels JGH (1994) Apical wall biogenesis. In: Wessels JGH & Meinhhardt F (Eds) The Mycota I (pp 125–141). Springer-Verlag, Berlin.
Sikkema J, de Bont JAM & Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59: 210–222.
Singh I, Lazo O, Dhaunsi GS & Contreras M (1992) Transport of fatty acids into human and rat peroxisomes. Differential transport of palmitic and lignoceric acids and its implication to X-adrenoleukodystrophy. J Biol Chem 267: 13306–133313.
Skatrud PL (1992) Genetic engineering of β-lactam antibiotic biosynthetic pathways in filamentous fungi. Trends Biotechnol 10: 324–329.
Skatrud PL, Tietz AJ, Ingolia TD, Cantwell CA, Fisher DL, Chapman JL & Queener SW (1989) Use of recombinant DNA to improve production of cephalosporin C by Cephalosporium acremonium. Bio/Technology 7: 476–485.
Skye GE & Segel IH (1970) Independent regulation of cysteine and cystine transport in Penicillium chrysogenum. Arch Biochem Biophys 138: 306–318.
Slayman CL (1987) The plasma membrane ATPase of Neurospora: a proton-pumping electroenzyme. J Bioenerg Biomembr 19: 1–20.
Slayman CL & Slayman CW (1974) Depolarization of the plasma membrane of Neurospora during active transportof glucose: evidence for a proton-dependent cotransport system. Proc Natl Acad Sci USA 71: 1935–1939.
Smith A (1985) Cephalosporins. In: Blanch HW, Drew S & Wang DIG (Eds) Comprehensive Biotechnology. The Principles, Applications and Regulations of Biotechnology in Industry, Agriculture and Medicine, Vol 3. The Practice of Biotechnology: Current Commodity Products (pp 163–185). Pergamon Press, Oxford.
Smith DJ, Burnham MKR, Edwards J, Earl AJ & Turner G (1990a) Cloning and heterologous expression of the penicillin biosynthetic gene cluster from Penicillium chrysogenum. Bio/Technology 8: 39–41.
Smith DJ, Earl AJ & Turner G (1990b) The multifunctional peptide synthetase performing the first step of penicillinbiosynthesis in Penicillium chrysogenum is a 421073 dalton protein similar to Bacillus brevis peptide antibiotic synthetases. EMBO J 9: 2743–2750.
Smith MW, Feng D-F & Doolittle RF (1992) Evolution by aquisition: the case of horizontal gene transfer. Trends Biochem Sci 17: 489–493.
Smith FW, Hawkesford MJ, Prosser IM & Clarkson DT (1995) Isolation of a cDNA from Saccharomyces cerevisiae that encodes a high affinity sulphate transporter at the plasma membrane. Mol Gen Genet 247: 709–715.
Sohn Y-S, Lee K-C, Koh Y-H & Gil G-H (1994) Changes in cellular fatty acid composition of Cephalosporium acremonium during cephalosporin C production. Appl Environ Microbiol 60: 947–952.
Sollner T & Rothman JE (1994) Neurotransmission: harnessing fusion machinery at the synapse. Trends Neurosci 17: 344–348.
Sophianopoulou V & Diallinas G (1995) Amino acid transporters of lower eukaryotes: regulation, structure and topogenesis. FEMS Microbiol Rev 16: 53–75.
Sousa MJ, Mota M & Leao C (1992) Transport of malic acid in the yeast Schizosaccharomyces pombe: Evidence for a proton-dicarboxylate symport. Yeast 8: 1025–1031.
Spallholz JE (1987) Glutathione: Is it an evolutionary vestige of the penicillins? Med hypotheses 23: 253–257.
Srikumar R, Li X-Z & Poole K (1997) Inner membrane efflux components are responsible for β-lactam specificity ofmultidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol 179: 7875–7881.
Stokes DL (1991) P-type ion pumps: structure determination may soon catch up with structure predictions. Curr Opin Struct Biol 1: 555–561.
Suárez T & Peñalva MA (1996) Characterization of a Penicillium chrysogenum gene encoding a PacC transcription factor and its binding sites in the divergent pcbAB-pcbC promoter of the penicillin biosynthetic cluster. Mol Microbiol 20: 529–540.
Subramani S (1993) Protein import into peroxisomes and biogenesis of the organelle. Annu Rev Cell Biol 9: 445–478.
Subramani S (1996) Protein translocation into peroxiomes. J Biol Chem 271: 32483–32486.
Sugawara M, Hashimoto A, Toda T, Takahashi M, Kobayashi M, Iseki K & Miyazaki K (1994a) Changes in the permeation rate of organic anions through the intestinal brush-border membrane with membrane surface potential. Biochim Biophys Acta 1190: 85–90.
Sugawara M, Hashimoto A, Kobayashi M, Iseki K & Miyazaki K (1994b) Effect of membrane surface potential on the uptake of anionic compounds by liposomes. Biochim Biophys Acta 1192: 241–246.
Sulter GJ, Harder W & Veenhuis M (1993a) Structural and functional aspects of peroxisomal membranes in yeasts. FEMS Microbiol Rev 11: 285–297.
Sulter GJ, Verheyden K, Mannaerts G, Harder W & Veenhuis M (1993b) The in vitro permeability of yeast peroxisomal membranes is caused by a 31 kDa integral membrane protein. Yeast 9: 733–742.
Suwalsky M, Villena F, Aguilar F & Sotomayor CP (1996) Interaction of penicillin G with the human erythrocyte membrane and models. Z. Naturforsch, C: Biosci 51: 243–248.
Swartz RW (1985) Penicillins. In: Blanch HW, Drew S & Wang DIC (Eds) Comprehensive Biotechnology. The Principles, Applications and Regulations of Biotechnology in Industry, Agriculture and Medicine, Vol 3. The Practice of Biotechnology: Current Commodity Products (pp 7–47). Pergamon Press, Oxford.
Swartzman EE, Viswanathan MN & Thorner J (1996) The PAL1 gene product is a peroxisomal ATP-binding cassette transporter in the yeast Saccharomyces cerevisiae. J Cell Biol 132: 549–563.
Szczypka MS, Wemmie JA, Moye-Rowley WS & Thiele DJ (1994) A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance associated protein. J Biol Chem 269: 22853–22857.
Tabak HF, Elgersma Y, Hettema E, Franse MM, Voorn-Brouwer T & Distel B (1995) Transport of proteins and metabolites across the impermeable membrane of peroxisomes. Cold Spring Harb Symp Quant Biol LX: 649–655.
Takeuchi Y, Schmid J, Caldwell JH & Harold FM (1988) Transcellular ion currents and extension of Neurospora crassa hyphae. J Membr Biol 101: 33–41.
Tan IKP, Fernández-Cañón JM, Reglero A & Luengo JM (1993) Effect of analogues of phenylacetic acid (PA) on the PA transport system in Penicillium chrysogenum strains HI 107 and M223. Appl Microbiol Biotechnol 40: 113–116.
Tanner W & Caspari T (1996) Membrane transport carriers. Annu Rev Plant Physiol 47: 595–626.
Tardrew PL & Johnson MJ (1958) Sulfate utilization by penicillin-producing mutants of Penicillium chrysogenum. J Bacteriol 76: 400–405.
Then Bergh K & Brakhage AA (1998) Regulation of the Aspergillus nidulans penicillin biosynthesis gene acvA (pcbAB) by amino acids: implication for involvement of transcription factor PACC. Appl Environ Microbiol 64: 843–849.
Theilgaard HBA, Kristiansen KN, Henriksen VM & Nielsen J (1997) Purification and characterization of δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine synthetase from Penicillium chrysogenum. Biochem J 327: 185–191.
Thieringer R & Kunau W-H (1991a) The β-oxidation system in catalase-free microbodies of the filamentous fungus Neurospora crassa. Purification of a multifunctional protein possessing 2-enoyl-CoA hydratase, L-3-hydroxyacyl-CoA dehydrogenase, and 3-hydroxyacyl-CoA epimerase activities. J Biol Chem 266: 13110–13117.
Thieringer R & Kunau W-H (1991b) β-Oxidation system of the filamentous fungus Neurospora crassa. Structural characterization of the trifunctional protein. J Biol Chem 266: 13118–3123.
Thomas D & Surdin-Kerjan Y (1997) Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 61: 503–532.
Tilburn J, Sakar S, Widdick DA, Espeso EA, Orejas M, Mungroo J, Peñalva MA & Arst HN Jr (1995) The Aspergillus PacC zinc finger transcription factor mediates regulation of both acidic-and alkaline-expressed genes by ambient pH. EMBO J 14: 779–790.
Tobin MB, Fleming MD, Skatrud PL & Miller JR (1990) Molecular characterization of the acyl-coenzyme A:isopenicillin N acyltransferase gene (penDE) from Penicillium chrysogenum and Aspergillus nidulans and activity of recombinant enzyme in Escherichia coli. J Bacteriol 172: 5908–5914.
Tobin MB, Kovacevic S, Madduri K, Hoskins JA, Skatrud PL, Vining LC, Stuttard C & Miller JR (1991) Localization of the lysine ε-aminotransferase (lat) and δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine synthetase (pcbAB) genes from Streptomyces clavuligerus and production of lysine ε-aminotransferase activity in Escherichia coli. J Bacteriol 173: 6223–6229.
Tobin MB, Baldwin JE, Cole SCJ, Miller JR, Skatrud PL & Sutherland JD (1993) The requirement for subunit interaction in the production of Penicillium chrysogenum acyl-coenzyme A:isopenicillin N acyltransferase in Escherichia coli. Gene 132: 199–206.
Tobin MB, Peery RB & Skatrud PL (1997) Genes encoding multiple drug resistance-like proteins in Aspergillus fumigatus and Aspergillus flavus. Gene 200: 11–23.
Torres NV, Riol-Cimas JM, Wolschek M & Kubicek CP (1996) Glucose transport by Aspergillus niger: the low-affinity carrier is only formed during growth on high glucose concentrations. Appl Microbiol Biotechnol 44: 790–794.
Traxler P, Treichler HJ & Nüesch J (1975) Synthesis of N-acetyldeacetoxycephalosporin C by a mutant of Cephalosporium acremonium. J Antibiot 28: 605–606.
Tweedie JW & Segel IH (1970) Specificity of transport processes for sulfur, selenium, and molybdenum anions by filamentous fungi. Biochim Biophys Acta 196: 95–106.
Unkles SE, Hawker KL, Grieve C, Campbell El, Montague P & Kinghorn JR (1991) crnA encodes a nitrate transporter in Aspergillus nidulans. Proc Natl Acad Sci USA 88: 204–208.
Usui S & Yu C-A (1989) Purification and properties of isopenicillin N epimerase from Streptomyces clavuligerus. Biochim Biophys Acta 999: 78–85.
Valenciano S, de Lucas JR, Pedregosa A, Monistrol IF & Laborda F (1996) Induction of β-oxidation enzymes andmicrobody proliferation in Aspergillus nidulans. Arch Microbiol 166: 336–341.
Valenciano S, de Lucas JR, van der Klei I, Veenhuis M & Laborda F (1998) Characterization of Aspergillus nidulans peroxisomes by immunoelectron microscopy. Arch Microbiol 170: 370–376.
van den Berg MA, Hillenga DJ & Bovenberg RAL (1998) Overexpression of the penicillin biosynthetic genes in Penicillium chrysogenum. In: Martín JF & Gutiérrez S (Eds) Fungal Genetics — Fourth European Conference on Fungal Genetics (p 202). Universidad de León, León
van den Bosch H, Schutgens RBH, Wanders RJA & Tager JM (1992) Biochemistry of peroxisomes. Annu Rev Biochem 61: 157–197.
van den Bossche H (1989) Importance and role of sterols in fungal membranes. In: Kuhn PJ, Trinci APJ, Jung MJ, Goosey MW & Copping LG (Eds) The Biochemistry of Cell Walls and Membranes in Fungi (pp 135–157). Springer-Verlag, Berlin.
van den Broek PJA, de Bruijne AW & van Steveninck J (1987) The role of ATP in the control of H+-galactoside symport in the yeast Kluyveromyces marxianus. Biochem J 242: 729–734.
van den Hazel HB, Kielland-Brandt MC & Winther JR (1996) Biosynthesis and function of yeast vacuolar proteases. Yeast 12: 1–16.
Vanderhaeghe H, Claesen M, Vlietinck A & Parmentier G (1968) Specificity of penicillin acylase of Fusarium and of Penicillium chrysogenum. Appl Microbiol 16: 1557–1563.
van der Rest ME, Kamminga AH, Nakano A, Anraku Y, Poolman B & Konings WN (1995) The plasma membrane of Saccharomyces cerevisiae: structure, function and biogenesis. Microbiol Rev. 59: 304–322.
van der Werf P, Griffith OW & Meister A (1975) 5-Oxo-L-prolinase (L-pyroglutamate hydrolase). Purification and catalytic properties. J Biol Chem 250: 6686–6692.
van Dijken JP & Veenhuis M (1980) Cytochemical localization of glucose oxidase in peroxisomes of Aspergillus niger. Eur J Appl Microbiol 9: 275–283.
Vanhoutte B, Pons MN, Thomas CR, Louvel L & Vivier H (1995) Characterization of Penicillium chrysogenum physiology in submerged cultures by color and monochrome image analysis. Biotechnol Bioeng 48: 1–11.
van Liempt H, von Döhren H & Kleinkauf H (1989) δ-(L-α-Aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. The first enzyme in penicillin biosynthesis is a multifunctional peptide synthetase J Biol Chem 264: 3680–3684.
van Roermund CWT, Elgersma Y, Singh N, Wanders RJA & Tabak HF (1995) The membrane of peroxisomes in Saccharomyces cervisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J 14: 3480–3486.
van Veen HW & Konings WN (1997) Multidrug transporters from bacteria to man: similarities in structure and function. Semin Cancer Biol 8: 183–191.
Van Veldhoven PP, Just WW & Mannaerts GP (1987) Permeability of the peroxisomal membrane to cofactors of β-oxidation. J Biol Chem 262: 4310–4318.
Veenstra AE, van Solingen P, Bovenberg RAL & van der Voort LHM (1991) Strain improvement of Penicillium chrysogenum by recombinant DNA techniques. J Biotechnol 17: 81–90.
Velasco J, Gutíerrez S, Fernández FJ, Marcos AT, Arenos C & Martín JF (1994) Exogenous methionine increases levels of mRNAs transcribed from pcbAB, pcbC and cefEF genes, encoding enzymes of the cephalosporin biosynthetic pathway, in Acremonium chrysogenum. J Bacteriol 176: 985–991.
Versaw WK (1995) A phosphate-repressible, high-affinity phosphate permease is encoded by the pho-5 + gene of Neurospora crassa. Gene 153: 135–139.
Versaw WK & Metzenberg RL (1995) Repressible cation-phosphate symporters in Neurospora crassa. Proc Natl Acad Sci USA 92: 3884–3887.
Wada Y & Anraku Y (1994) Chemiosmotic coupling of ion transport in the yeast vacuole: its role in acidification inside organelles. J Bioenerg Biomembr 26: 631–637.
Wada Y, Ohsumi Y, Tanifuji M, Kasai M & Anraku Y (1987) Vacuolar ion channel of the yeast, Saccharomyces cerevisiae. J Biol Chem 262: 17260–17263.
Walton PA, Hill PE & Subramani S (1993) Import of stably folded proteins into peroxisomes. Mol Cell Biol 6: 675–683.
Wanner G & Theimer RR (1982) Two types of microbodies in Neurospora crassa. Ann NY Acad Sci 286: 269–284.
Waterham HR & Cregg JM (1996) Peroxisome biogenesis. BioEssays 19: 57–66.
Weil J, Miramonti J & Ladisch MR (1995) Biosynthesis of cephalosporin C: Regulation and recombinant technology. Enz Microbial Technol 17: 88–90.
Wemmie JA, Szczypka MS, Thiele DJ & Moye-Rowley WS (1994) Cadmium tolerance mediated by the yeast AP-1 protein requires the presence of an ATP-binding cassette transporter-encoding gene, YCF1. J Biol Chem 269: 35592–35597.
Whiteman PA, Abraham EP, Baldwin JE, Fleming MD, Schofield CJ, Sutherland JD & Willis AC (1990) Acyl coenzyme A:6-aminopenicillanic acid acyltransferase from Penicillium chrysogenum and Aspergillus nidulans. FEES Lett 262: 342–344.
Yamaguchi A, Hiruma R & Sawai T (1982) Phospholipid bilayer permeability of β-lactam antibiotics. J Antibiot 35: 1692–1699.
Yamamoto LA & Segel IH (1966) The inorganic sulfate transport system of Penicillium chrysogenum. Arch Biochem Biophys 114: 523–538.
Yeh W-K, Dotzlaf JE & Huffman GW (1991) Biochemical characterization and evolutionary implication of β-lactam expandase/hydroxylase, expandase and hydroxylase. In: Kleinkauf H & von Döhren H (Eds) 50 Years of Penicillin Application — History and Trends (pp 208–223). Public, Czech republic.
Yompakdee C, Bun-ya M, Shikata K, Ogawa N, Harashima S & Oshima (1996) A putative new membrane protein, Pho86p, in the organic phosphate uptake system of Saccharomyces cerevisiae. Gene 171: 41–47.
Zhang J & Demain AL (1992a) ACV synthetase. Crit Rev Biotechnol 12: 245–260.
Zhang J & Demain AL (1992b) Regulation of ACV synthetase activity in the β-lactam biosynthetic pathway by carbon sources and their metabolites. Arch Microbiol 158: 364–369.
Zhang J, Wolfe S & Demain AL (1988) Phosphate repressible and inhibitable β-lactam synthetases in Cephalosporium acremonium strain C-10. Appl Microbiol Biotechnol 29: 242–247.
Zhang J, Wolfe S & Demain AL (1992) Biochemical studies on the activity of the δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine synthetase from Streptomyces clavuligerus. Biochem J 283: 691–698.
Zinser E & Daum G (1995) Isolation and biochemical characterization of organelles from the yeast, Saccharomyces cerevisiae. Yeast 11: 493–536.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van de Kamp, M., Driessen, A.J. & Konings, W.N. Compartmentalization and transport in β-lactam antibiotic biosynthesis by filamentous fungi. Antonie Van Leeuwenhoek 75, 41–78 (1999). https://doi.org/10.1023/A:1001775932202
Issue Date:
DOI: https://doi.org/10.1023/A:1001775932202