Abstract
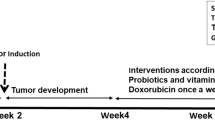
The present study was conducted to ascertain the modulatory influences of Ferula asafoetida L. (asafoetida, flavoring agent) on the mammary epithelial tissue differentiation, hepatic drug metabolizing enzymes, antioxidant profiles and N-methyl-N-nitrosourea (MNU)-induced mammary carcinogenesis in Sprague-Dawley rats. Feeding with two doses of asafoetida (1.25 and 2.5% w/w in diet) showed a remarkable increase in the development and differentiation of ducts/ductules (p < 0.01–0.005), lobules (p < 0.005) and a decrease in terminal end buds (p < 0.05–0.005) as compared to both normal and MNU-treated control animals. To assess the biochemical parameters, effect of asafoetida on drug-metabolizing enzymes was evaluated in the liver of rats. Asafoetida treatment significantly reduced (p < 0.05) the levels of cytochrome P450 and b5. There was an enhancement in the activities of glutathione S-transferase (p < 0.05–0.005), DT-diaphorase (p < 0.05–0.01), superoxide dismutase (p < 0.01–0.005) and catalase (p < 0.05–0.005) and in the level of reduced glutathione (p < 0.05–0.005), followed by asafoetida treatment. Also, asafoetida significantly restored the level of antioxidant system, depleted by MNU-treatment. The strengthening of antioxidant system by the lower and higher doses of asafoetida in the presence and absence of MNU was further substantiated by a significant inhibition (p < 0.005) in lipid peroxidation as measured by thiobarbituric acid-reactive substances (TBARS) in the liver of rat. Further, in long-term animal studies, where MNU was used to induce mammary carcinogenesis, asafoetida treatment resulted in a significant reduction in the multiplicity (p < 0.001) and size of palpable mammary tumors (p < 0.005–0.001) and a delay in mean latency period of tumor appearance (p < 0.005). Together, these findings indicate the chemopreventive potential of asafoetida against MNU-induced mammary carcinogenesis. Thus, asafoetida needs further investigation with regard to identification and characterization of its active principle(s) and mechanism of action, for this compound to be developed as a potential chemopreventive agent for human cancers.
Similar content being viewed by others
References
WHO: TheWorld Health Report. Geneva, 1997
Brown PH, Lippman SM: Chemoprevention of breast cancer. Breast Cancer Res Treat 62: 1–17, 2000
Chamberlain DG: Identity of Ferula asafetida: Notes. Royl Bot Gdn, Edib, 35: 229–233, 1977
Clark R: Animal models of breast cancer: their diversity and role of biomedical research. Breast Caner Res Treat 39: 1–6, 1996
Gullino PM, Pettigrew HM, Grantham FH: N-nitrosomethylurea as mammary gland carcinogen in rats. J Natl Cancer Inst Monogr 54: 401–414, 1975
McCormick DL, Adamowski CB, Fiks A, Moon RC: Lifetime dose response relationships for mammary tumor induction by a single administration of N-methyl-N-nitrosourea. Cancer Res 41: 1690–1694, 1981
Thompson HJ, Adlakha H: Dose-responsive induction of mammary gland carcinomas by the intraperitoneal injection of 1-methyl-1-nitrosourea. Cancer Res 51: 3411–3415, 1991
Banerjee MR: Response to mammary cells to hormones. Int Rev Cytol 47: 1–84, 1976
Omura T, Sato R: The carbon monoxide binding pigment of liver microsomes. J Biol Chem 239: 2370–2378, 1964
Moron MA, Depierre JW, Mannervik B: Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582: 67–78, 1979
Habig WH, Pabst MJ, Jacoby WB: Glutathione-S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139, 1974
Ernster L, Danielson L, Ljunggren M: DT-diaphoreses. I. Puri-fication from soluble fraction of rat liver cytoplasm. Biochim Biophys Acta 58: 171–188, 1962
Aebi H: Catalase in vitro. In: Colowick SP, Kaplan NO (eds) Methods in Enzymology. Academic Press, New York, 1984, pp 121–126
Marklund S, Marklund G: Involvement of superoxide anion radical in autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469–474, 1974
Varshney R, Kale RK: Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 58: 733–743, 1990
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with folin phenol reagent. J Biol Chem 193: 265–275, 1951
Russo IH, Russo J: Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz(a)anthracene. J Nat Can Inst 61: 1439–1449, 1978
Russo IH, Tewari M, Russo J: Morphology and development of the ratmammary gland. Monograph Series on the Pathology of Laboratory Animals. Springer Verlag, Berlin, 1988
Marginson GP, O'Connor PJ: Nucleic acid modification by N-nitroso compounds. In: Grover PL (ed.) Chemical Carcinogens and DNA. CRC Press, Boca Raton, FL, 1979, pp 111–159
O'Connor PJ, Saffhill R, Marginson GP: N-nitroso compounds: biochemical mechanisms of action. In: Emmolet P, Kreik E (eds) Environmental Carcinogenesis, Occurrence and Risk Evaluation and Mechanisms. Elsevier/North-Holland/Biochemical Press, Amsterdam, 1979, pp 73–96
Pegg AE: Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res 25: 195–269, 1977
Mori H, Kawabata K, Matsunaga K, Ushida J, Fujii K, Hara A, Tanaka T, Murai H: Chemopreventive effects of coffee bean and rice constituents on colorectal carcinogenesis. Biofactors 12: 101–105, 2000
Wargovich MJ, Jimenez A, McKee K, Steele VE, Velasco M, Woods J, Price R, Gray K, Kelloff GJ: Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis 21: 1149–1155, 2000
Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A: Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev 9: 1163–1170, 2000
Hirose M, Takahashi S, Ogawa K, Futakuchi M, Shirai T, Shibutani M, Uneyama C, Toyoda K, Iwata H: Chemoprevention of heterocyclic amine-induced carcinogenesis by Phenolic compounds in rats. Cancer Lett 143: 173–199, 1999
Tanaka T, Kojima T, Kawamori T, Wang A, Suzui M, Okamoto K, Mori H: Inhibition of 4-nitroquinoline-1-oxide-induced rat carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogenesis 14: 13211–13215, 1993
Murakami A, Wada K, Ueda N, Sasaki K, Haga M, Kuki W, Takahashi Y, Yonei H, Koshimizu K, Ohigashi H: In vitro absorption and metabolism of a citrus chemopreventive agent, auraptene, and its modifying effects on xenobiotic enzyme activities in mouse livers. Nutr Cancer 36: 191–199, 2000
Aruna K, Sivaramakrishnan VM: Plant products as protective agents against cancer. Indian J Exp Biol 28: 1008–1011, 1990
Abakumova OIu, Kutsenko NG, Lerman MI: Metabolic activation of N-methyl-N-nitrosourea in normal and tumor cells. Biokhimiia 43: 2092–2099, 1978
Prochaska HJ, Talalay P: Role of NAD(P)H: quinone reductase in protection against the toxicities of quinones and related agents, in oxidative stress. In: Sies H (ed.) Oxidants and Antioxidants. Academic Press, London, 1991, 195 pp
Cengiz M, Hacihanefioglu S, Cenani A: Glutathione and related enzymes in rat liver treated with methyl nitrosourea. J Basic Clin Physiol Pharmacol 7: 341–345, 1996
Bishayee A, Oinam S, Basu M, Chatterjee M: Vanadium chemoprevention of 7,12-dimethylbenz (a) anthraceneinduced rat mammary carcinogenesis: probable involvement of representative hepatic phase I and II xenobiotic metabolizing enzymesz. Breast Cancer Res Treat 63: 133–145, 2000
Kaplowitz N, Tsukamoto H: Oxidative stress and liver disease. Progress in Liver Diseases 14: 131–159, 1996
Saleem M, Alam A, Sultana A, Saleem S: Asafoetida inhibits early events of carcinogenesis: a chemopreventive study. Life Sci 68: 1913–1921, 2001
Unnikrishnan MC, Kuttan R: Tumour reducing and anticarcinogenic activity of selected spices. Cancer Lett 51: 85–89, 1990
Rana MP, Ghosh R, Chatterjee M: Change in tissue levels in glutathione, extent of lipid peroxidation and activity of glutathione peroxidase in transplantable murine lymphoma. Oncology 5: 25–29, 1994
Sarkar A, Bishayee A, Chatterjee M: Beta-carotene prevents lipid peroxidation and red blood cell membrane protein damage in experimental hepatocarcinogenesis. Cancer Biochem Biophys 15: 115–125, 1995
Welsch CW, Brown CK, Goodrich-Smith M, Chuisano J, Moon RC: synergistic effect of chronic prolactin suppression and retinoid treatment in the prophylaxis of N-methyl-N-nitrosourea induced mammary tumorigenesis in female Sprague-Dawley rats. Cancer Res 40: 3095–3098, 1980
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mallikarjuna, G., Dhanalakshmi, S., Raisuddin, S. et al. Chemomodulatory Influence of Ferula asafoetida on Mammary Epithelial Differentiation, Hepatic Drug Metabolizing Enzymes, Antioxidant Profiles and N-methyl-N-Nitrosourea-Induced Mammary Carcinogenesis in Rats. Breast Cancer Res Treat 81, 1–10 (2003). https://doi.org/10.1023/A:1025448620558
Issue Date:
DOI: https://doi.org/10.1023/A:1025448620558