Abstract
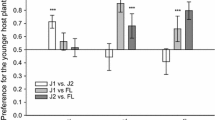
Damage by larvae of the buckeye butterfly (Junonia coenia) resulted in removal of 15–25% of Plantago lanceolata leaf area. Plants grown under high nutrients were larger than those grown under low nutrients. Twenty-eight days after herbivory, plants grown under high nutrients were still larger than those grown under low nutrients, and plants exposed to herbivores were significantly smaller than those not exposed to herbivores, regardless of the nutrient treatment. Damage by larvae also increased the iridoid glycoside content in the leaves and reproductive tissues of these Plantago lanceolata relative to undamaged controls. Whether damaged or undamaged, the iridoid glycoside content of P. lanceolata was highest in the reproductive tissues and lowest in the roots. Although initial concentrations of iridoid glycosides were significantly higher in plants grown under low nutrient conditions than in plants grown under high nutrient conditions, nutrient availability did not alter the phytochemical response of plants to herbivore damage. These results provide additional support for the defensive role of the iridoid glycosides in Plantago lanceolata by demonstrating that phytochemical variation is not always an incidental effect of nutrient stress but can be a direct response to damage by herbivores.
Similar content being viewed by others
REFERENCES
Baldwin, I. T. 1988. Short-term damage-induced alkaloids protect plants. Oecologia 75:367-370.
Baldwin, I. T., Oesch, R. C., Merhige, P. M., and Hayes, K. 1993. Damage-induced root nitrogen metabolism in Nicotiana sylvestris: Testing C/N predictions for alkaloid production. J. Chem. Ecol. 19:3029-3043.
Bazzaz, F. A., Chiariello, N. R., Coley, P. D., and Pitelka, L. F. 1987. Allocating resources to reproduction and defense. Bioscience 37:58-67.
Bowers, M. D. 1984. Iridoid glycosides and hostplant specificity in larvae of the buckeye butterfly, Junonia coenia Nymphalidae. J. Chem. Ecol. 10:1567-1577.
Bowers, M. D., and Puttick, G. M. 1988. The effect of qualitative variation in iridoid glycosides on generalist and specialist lepidopteran herbivores. J. Chem. Ecol. 14:319-334.
Bowers, M. D., and Puttick, G. M. 1989. Iridoid glycosides and insect feeding preferences: Gypsy moths, Lymantria dispar Lymantriidae, and buckeyes, Junonia coenia Nymphalidae. Ecol. Entomol. 14:247-256.
Bowers, M. D., and Stamp, N. E. 1993. Effects of plant age, genotype and herbivory on Plantago performance and chemistry. Ecology 74:1778-1791.
Bryant, J. P., Chapin, F. S., and Klein, D. R. 1983. Carbon/nutrient balance of boreal plants in the role of plant chemistry. Annu. Rev. Ecol. Syst. 11:261-285.
Bryant, J. P., Tuomi, J., and NiemelÄ, P. 1988. Environmental constraint of constitutive and long-term inducible defenses in woody plants, pp. 367-389, in K. C. Spencer (ed.). Chemical Mediation of Coevolution. Academic Press, San Diego.
Bryant, J. P., Heitkonig, I., Kuropat, P., and Owen-Smith, N. 1991. Effects of severe defoliation on the long-term resistance to insect attack and on leaf chemistry in six woody species of the southern African savanna. Am. Nat. 137:50-63.
Bryant, J. P., Reichardt, P. B., Clausen, T. P., and Werner, R. A. 1993. Effects of mineral nutrition on delayed inducible resistance in Alaska paper birch. Ecology 74:2072-2084.
Cavers, P. B., Bassett, I. J., Crompton, C. W. 1980. The biology of Canadian weeds. 47. Plantago lanceolata L. Can. J. Plant Sci. 60:1269-1282.
Chapin, F. S. 1980. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 11:233-260.
Clausen, T. P., Reichardt, P. B., Bryant, J. P., and Werner, R. A. 1991. Long-term and short-term induction in quaking aspen: Related phenomena? pp. 71-84, in D. W. Tallamy and M. J. Raupp (eds.). Phytochemical Induction by Herbivores. Wiley, New York.
Dixon, R. A., Bailey, J. A., Bell, J. N., Bolwell, G. P., Cramer, C. L., Edwards, K., Hamden, M. A. M. S., Lamb, C. J., Robbins, M. P., Ryder, T. B., and Schuch, W. 1986. Rapid changes in gene expression in response to microbial elicitation. Phil. Trans. Soc. London B 314:411-426.
Edwards, P. J., Wratten, S. D., and Cox, H. 1986. Wound-induced changes in the acceptability of tomato to larvae of Spodoptera litteralis. A laboratory bioassay. Ecol. Entomol. 10:155-158.
Faeth, S. H. 1986. Indirect interactions between temporally separated herbivores mediated by the host plant. Ecology 67:479-494.
Fajer, E. D., Bowers, M. D., and Bazzaz, F. A. 1992. The effect of nutrients and enriched CO2 environments on production of carbon-based allelochemicals in Plantago: A test of the carbon/nutrient balance hypothesis. Am. Nat. 140:707-723.
Gibberd, R., Edwards, P. J., and Wratten, S. D. 1988. Wound-induced changes in the acceptability of tree foliage to Lepidoptera: Within-leaf effects. Oikos 51:43-47.
Haukioja, E. 1980. On the role of plant defenses in the fluctuation of herbivore populations. Oikos 35:202-213.
Haukioja, E., and Niemela, P. 1977. Retarded growth of geometrid larva after mechanical damage of its host tree. Ann. Zool. Fenn. 14:48-52.
Haukioja, E., and Neuvonen, S. 1985. Induced long-term resistance of birch foliage against defoliators: Defensive or incidental? Ecology 66:1303-1308.
Haukioja, E., Suomela, J., and Neuvonen, S. 1985. Long-term inducible resistance in birch foliage: Triggering cues and efficacy on a defoliator. Oecologia 65:363-369.
Herms, D. A. and Mattson, W. J. 1992. The dilemma of plants: To grow or defend. Q. Rev. Biol. 67:283-335.
Hugentobler, U., and Renwick, J. A. A. 1995. Effects of plant nutrition on the balance of insect relevant cardenolides and glucosinolates in Erysimum cheriranthoides. Oecologia 102:95-101.
Hunter, M. D., and Schultz, J. C. 1995. Fertilization mitigates chemical induction and herbivore responses within damaged oak trees. Ecology 76:1226-1232.
Karban, R. and Baldwin, I. T. 1997. Induced Responses to Herbivory. Chicago University Press, Chicago.
Karban, R., and Meyers, J. H. 1989. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 20:331-348.
Maschinski, J. and Whitham, T. 1989. The continuum of plant responses to herbivory: The influence of plant association, nutrient availability, and timing. Am. Nat. 134:1-19.
McKey, D. 1974. Adaptive patterns in alkaloid physiology. Am. Nat. 108:305-320.
McKey, D. 1979. The distribution of secondary compounds within plants, pp. 55-133, in G. A. Rosenthal and D. H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Neuvonen, S., and Haukioja, E. 1984. Low nutritive quality as defense against herbivores: Induced responses in birch. Oecologia 63:71-74.
Pereyra, P. C., and Bowers, M. D. 1988. Iridoid glycosides as oviposition stimulants for the buckeye butterfly, Junonia coenia Nymphalidae. J. Chem. Ecol. 14:917-928.
Primack, R. B., and Antonovics, J. 1982. Experimental ecological genetics in Plantago. VII. Reproductive effort in populations of P. lanceolata L. Evolution 36:742-752.
Puttick, G. M., and Bowers, M. D. 1988. The effect of iridoid glycosides on growth, survival, and food choice of the armyworm, Spodoptera eridania Lepidoptera: Noctuidae. J. Chem. Ecol. 14:335-351.
Rhoades, D. F. 1979. Evolution of plant chemical defenses against herbivory, pp. 3-54, in G. A. Rosenthal and D. H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Schultz, J. C., and Baldwin, I. T. 1982. Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149-151.
Scott, J. A. 1986. The Butterflies of North America. Standard University Press, Stanford, California.
Scriber, J. and Slansky, F. 1981. The nutritional ecology of immature insects. Annu. Rev. Entomol. 26:183-211.
Stamp, N. E., and Bowers, M. D. 1996. Consequences for plantain chemistry and growth when herbivores are attacked by predators. Ecology 72:535-549.
Stout, M. J., and Duffey, S. S. 1996. Characterization of induced resistance in tomato plants. Entomol. Exp. Appl. 79:273-283.
Stout, M. J., Brovont, R. A., and Duffey, S. S. 1998. Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. J. Chem. Ecol. 24:945-963.
Tallamy, D. W. 1985. Squash beetle feeding behavior: An adaptation against induced cucurbit defenses. Ecology 66:1574-1579.
Tuomi, J., NiemelÄ, P., Haukioja, E., and Neuvonen, S. 1984. Nutrient stress: An explanation for plant anti-herbivore responses to defoliation. Oecologia 61:208-210.
Tuomi, J., NiemelÄ, P., Chapin, F. S., Bryant, J. P., and SirÉn, S. 1988. Defensive responses of trees in relation to their carbon/nutrient balance, pp. 57-72 in W. J. Mattson, J. Levieux, and C. Bernard-Dagan (eds.). Mechanisms of Woody Plant Defenses against Insects: Search for Pattern. Springer-Verlag, New York.
Tuomi, J., NiemelÄ, P., and SirÉn, S. 1990. The panglossian paradigm and delayed inducible accumulation of foliar phenolics in mountain birch. Oikos 59:399-410.
Wilkens, R. T., Spoerke, J. M., and Stamp, N. E. 1996. Differential responses of growth and two soluble phenolics of tomato to resource availability. Ecology 77:247-258.
Zangerl, A. R. 1990. Furanocoumarin induction in wild parsnip: Evidence for an induced defense against herbivores. Ecology 71:1926-1932.
Zangerl, A. R., and Berenbaum, M. R. 1995. Spatial, temporal, and environmental limits on xanthotoxin induction in wild parsnip foliage. Chemoecology 1:37-42.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Darrow, K., Bowers, M.D. Effects of Herbivore Damage and Nutrient Level on Induction of Iridoid Glycosides in Plantago lanceolata . J Chem Ecol 25, 1427–1440 (1999). https://doi.org/10.1023/A:1020991229072
Issue Date:
DOI: https://doi.org/10.1023/A:1020991229072