Abstract
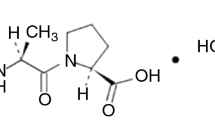
The drug-excipient incompatibility screen for moexipril hydrochloride (1) using various isothermal stress methods is reported herein. It was found that most of the commonly used fillers, disintegrants, lubricants, glidants, and coating agents were incompatible with 1 in dry powder mixtures; moisture and basic (or alkalizing) agents were determined to be the dominant destabilizing factors. In wet granulations, basic agents, however, were found to suppress drug degradation even in the presence of moisture. Supported by the product distribution studies, the stabilization is proposed to involve the neutralization of the acidic drug by the basic excipients.
Similar content being viewed by others
REFERENCES
D. C. Monkhouse and J. L. Lach. Can. J. Pharm. Sci. 7:29–46 (1972).
J. T. Carstensen. J. Pharm. Sci. 63:1–14 (1974).
H. H. El-Shattawy, D. O. Kildsig, and G. E. Peck. Drug Dev. Ind. Pharm. 8:923–935 (1982).
H. H. El Shattawy. Drug Dev. Ind. Pharm. 8:819–831 (1982).
E. C. Signoretti, A. Dell'Utric, A. D. Salvo, and L. Donini. Drug Dev. Ind. Pharm. 12:603–620 (1986).
A. A. Van Dooren. Drug Dev. Ind. Pharm. 9:43–55 (1983).
F. A. Chrzanowski, L. A. Ullissi, B. J. Fegely, and A. C. Newman. Drug Dev. Ind. Pharm. 12:783–800 (1986).
M. J. Wyvratt and A. A. Patchett. Med. Res. Rev. 5:485–531 (1985).
P. K. Shiromani and J. F. Bwitz. Drug Dev. Ind. Pharm. 12:2467–2480 (1986).
M. L. Cotton, D. W. Wu, and E. B. Vadas. Inst. J. Pharm. 40:129–142 (1987).
L. Gu and R. G. Strickley. Pharm. Res. 4:392–397 (1987).
L. Gu and R. G. Strickley. Pharm. Res. 5:765–771 (1988).
R. E. Bouvette and G. A. Digenis. Pharm. Res. 4:5–35 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gu, L., Strickley, R.G., Chi, LH. et al. Drug-Excipient Incompatibility Studies of the Dipeptide Angiotensin-Converting Enzyme Inhibitor, Moexipril Hydrochloride: Dry Powder vs Wet Granulation. Pharm Res 7, 379–383 (1990). https://doi.org/10.1023/A:1015871406549
Issue Date:
DOI: https://doi.org/10.1023/A:1015871406549