Abstract
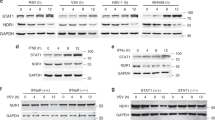
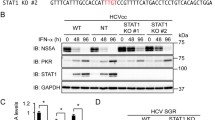
Interferons are a family of cytokines that exerts antiviral, antitumor and immunomodulatory actions by inducing a complex set of proteins. One of the best known IFN-induced protein is the dsRNA-dependent protein kinase (PKR), that mediates both antiviral and anticellular activities. PKR inhibits translation initiation through the phosphorylation of the alpha subunit of the initiation factor eIF-2 (eIF-2α) and also controls the activation of several transcription factors such as NF-κB, p53, or STATs. In addition, PKR mediates apoptosis induced by many different stimuli, such as treatment with LPS, TNF-α, viral infection, or serum starvation. The mechanism of apoptosis induction by PKR involves phosphorylation of eIF-2α and activation of NF-κB. In this way, expression of different genes is regulated by PKR. Among the genes upregulated in response to PKR are Fas, Bax and p53. The pathway of PKR-induced apoptosis involves FADD activation of caspase 8 by a mechanism independent of Fas and TNFR. Since IFNs are used as drugs for different disorders such as viral infection and cancer, understanding the pathway of apoptosis induction triggered by PKR should be useful in the rational design of IFN therapies.
Similar content being viewed by others
References
Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem 1998; 67: 227–264.
Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci USA 1998; 95: 15623–15628.
Meurs EF, Chong K, Galabru J, et al. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 1990; 62: 379–390.
Patel RC, Sen GC. PACT, a protein activator of the interferoninduced protein kinase, PKR. EMBO J 1998; 17: 4379–4390.
Ito T, Yang M, May S. RAX, a cellular activator for doublestranded RNA dependent protein kinase during stress signaling. J Biol Chem 1999; 274: 15427–15432.
Clemens MJ, Elia A. The double-stranded RNA-dependent protein kinase PKR: Structure and function. J Interferon Cytokine Res 1997; 17: 503–524.
Levin D, London IM. 1978. Regulation of protein synthesis: Activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci USA 1978; 75: 1121–1125.
Kumar A, YangY, FlatiV, et al. Deficient cytokine signalling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: Role of IRF-1 and NF-B. EMBO J 1997; 16: 406–416.
Cuddihy AR, Li S, Tam NWN, Wong AH, et al. Doublestranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol Cell Biol1999; 19: 2475–2484.
Cuddihy AR, Wong AH, Tam NWN, Li S, Koromilas AE. The double-stranded-RNA-activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene 1999; 18: 2690–2702.
Wong AH, Tam NW, Yang YL, et al. Physical-association between STAT1 and the interferon-inducible protein kinase PKRand implications for interferon and double-strandedRNA signaling pathways. EMBO J 1997; 16: 1291–1304.
Langland JO, Kao PN, Jacobs BL. Nuclear Factor-90 of activated T-cells: A double-stranded RNA binding protein and substrate for the double-stranded RNA-dependent protein kinase, PKR. Biochemistry 1999; 38: 6361–6368.
Patel RC, Vestal DJ, Xu Z, et al. DRBP76, a doublestranded RNA-binding nuclear protein, is phosphorylated by the interferon-induced protein kinase, PKR. J Biol Chem1999; 274: 20432–20437.
Chong KL, Feng L, Schappert K, et al. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J 1992; 11: 1553–1562.
Petryshyn R, Chen JJ, London IM. Growth-related expression of a double-stranded RNA-dependent protein kinase in 3T3 cells. J Biol Chem 1984; 259: 14736–14742.
Meurs E, Galabru J, Barber GN, Katze MG, Hovanessian AG. Tumor suppressor function of the interferon-induced doublestranded RNA-acvtivated protein kinase. Proc Natl Acad Sci USA 1993; 90: 232–236.
Koromilas AE, Galabru J, Barber GN, Katze MG, Sonenberg N. Malignant trasformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science 1992; 257: 1685–1689.
Lee SB, Esteban M. The interferon-induced double-stranded RNA-activated human p68 protein kinase inhibits the replication of vaccinia virus. Virology 1993; 193: 1037–1041.
Lee SB, Bablanian R, Esteban M. Regulated expression of the interferon-induced protein kinase p68 (PKR) by vaccinia virus recombinants inhibits the replication of vesicular stomatitis virus but not that of poliovirus. J Interferon Cytokine Res 1996; 16: 1073–1078.
Zamanian-Daryoush M, Der SD, Williams BR. Cell cycle regulation of the double stranded RNA activated protein kinase, PKR. Oncogene 1999; 18: 315–326.
Lee SB, Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology1994; 199: 491–496.
Barber GN, Thompson S, Lee TG, et al. The 58–kilodalton inhibitor of the interferon-induced double-stranded RNAactivated protein kinase is a tetraticopeptide repeat protein with oncogenic potential. Proc Natl Acad Sci USA 1994; 91: 4278–4282.
Wu S, Rehemtulla A, Gupta NK, Kaufman RJ. A eukaryotic translation initiation factor 2–associated 67 kDa glycoprotein partially reverses protein synthesis inhibition by activated double-stranded RNA-dependent protein kinase in intact cells. Biochemistry 1996; 35: 8275–8280.
Park H, Davies MV, Langland JO, et al. TAR RNA-binding protein is an inhibitor of the interferon-induced protein kinase PKR. Proc Natl Acad Sci USA 1994; 91: 4713–4717.
Benkirane M, Neuveut C, Chun Rf, et al. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. EMBO J 1997; 16: 611–624.
Gale M Jr, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther 1998; 78: 29–46.
Katze MG, Decorato D, Safer B, Galabru J, Hovanessian AG. Adenovirus VAI RNA complexes with the 68,000 Mr protein kinase to regulate its autophosphorylation and activity. EMBO J 1987; 6: 689–697.
Davies MV, ChangHW, Jacobs BL, Kaufman RJ. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J Virol 1993; 66: 1688–1692.
Lloyd RM, Shatkin AJ. Translational stimulation by reovirus polypeptide sigma 3: Substitution forVAIRNAand inhibition of phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Virol 1993; 66: 6878–6884.
Gale M Jr, Blakely CM, Kwieciszewski B, et al. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: Molecular mechanisms of kinase regulation. Mol Cell Biol 1998; 18: 5208–5218.
Gale MJ Jr, Korth MJ, Tang NM, et al. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 1997; 230: 217–227.
Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 1999; 285: 107–110.
Dever TE, Sripriya R, McLachlin JR, et al. Disruption of cellular translational control by a viral truncated eukaryotic translation initiation factor 2alpha kinase homolog. Proc Natl Acad Sci USA 1998; 95: 4164–4169.
He B, Gross M, Roizman B. The ° 1 34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1® to dephosphorylate the ® subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA 1997; 94: 843–848.
Black TL, Safer B, Hovanessian A, Katze MG. The cellular 68,000–Mr protein kinase is highly autophosphorylated and activated yet significantly degraded during poliovirus infection: Implications for translational regulation. J Virol 1989; 63: 2244–2251.
Kibler KV, Shors T, Perkins KB, et al. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J Virol 1997; 71: 1992–2003.
Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem 1998; 273: 2416–2423.
Der SD, Yang Y, Weissmann C, Williams BRG. A doublestranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA1997; 94: 3279–3283.
Jacobs BL, Langland JO. When two strands are better than one: The mediators and modulators of the cellular responses to double-stranded RNA. Virology 1996; 219: 339–349.
Díaz-Guerra M, Rivas C, Esteban M. Activation of the IFNinducible enzyme RNase L causes apoptosis of animal cells. Virology 1997; 236: 354–363.
Castelli JA, Hassel BA, Wood KA, et al. A study of the interferon antiviral mechanism: Apoptosis activation of the 2–5A system. J Exp Med 1997; 186: 967–972.
Zhou A, Paranjape J, Brown TL, et al. Interferon action and apoptosis are defective in mice devoid of 2',5'-oligoadenylatedependent RNase L. EMBO J 1997; 16: 6355–6363.
Rivas C, Gil J, Melkova Z, Esteban M, Diaz-Guerra M. Vaccinia virus E3L protein is an inhibitor of the interferon (IFN)-induced 2–5A synthetase enzyme. Virology 1998; 243: 406–414.
Wada N, Matsumura M, Ohba Y, Kobayashi N, Takizawa T, Nakanishi Y. Transcription stimulation of the Fas-encoding gene by nuclear factor for Interleukin-6 expression upon influenza virus infection. J Biol Chem 1995; 270: 18007–18012.
Takizawa T, Ohashi K, Nakanishi Y. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J Virol 1996; 70: 8128–8132.
Yeung MC, Chang DL, Camantigue RE, Lau AS. Inhibitory role of the host apoptogenic gene PKR in the establishment of persistent infection by encephalomyocaritis virus in U937 cells. Proc Natl Acad Sci USA 1999; 96: 11860–11865.
Kaufman RJ. Double-stranded RNA-activated protein kinase mediates virus-induced apoptosis: A new role for an old actor. Proc Natl Acad Sci USA 1999; 96: 11693–11695.
Yeung MC, Liu J, Lau A. An essential role for the interferoninducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci USA 1996; 93: 12451–12455.
Ito T, Jagus R, May WS. Interleukin 3 stimulates protein synthesis by regulating double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci USA 1994; 91: 7453–7459.
Alcazar A, Bazan F, Rivera J, Salinas M. Phosphorylation of initiation factor 2® subunit and apoptosis in Ca2+ionophoretreated cultured neuronal cells. Neurosci Lett 1995; 201: 215–218.
Datta B, Datta R. Induction of apoptosis due to lowering the level of eukaryotic initiation factor 2–associated protein, p67, from mammalian cells by antisense approach. Exp Cell Res 1999; 246: 376–383.
Tang NM, Korth MJ, Gale M Jr., et al. Inhibition of doublestranded RNA-and tumor necrosis factor alpha-mediated apoptosis by tetraticopeptide repeat protein and cochaperine p58IPK. Mol Cell Biol 1999; 19: 4757–4765.
Hershey JW. Translational control in mammalian cells. Annu Rev Biochem 1991; 60: 717–755.
Gil J, Alcamí J, Esteban M. Induction of apoptosis by doublestranded-RNA-dependent protein kinase (PKR) involves the ® subunit of eukaryotic translation initiation factor 2 and NF-·B. Mol Cell Biol 1999; 19: 4653–4663.
Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-·B and AP-1. Mol Cell 1998; 1: 543–551.
Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: Gene structure, chromosomal localization and species specificity. Int Immunol 1994; 6: 1567–1574.
Behrmann I, Walczac H, Krammer PH. Structure of the human APO-1 gene. Eur J Immunol 1994; 24: 3057–3062.
Casano FJ, Rolando AM, Mudgett US, Molineaux SM. The structure and complete nucleotide sequence of the murine gene encoding interleukin-1 beta converting enzyme (ICE). Genomics1994; 20: 474–481.
Wu H, Lozano G. NF-·B activation of p53. A potential mechanism for suppressing cell growth in response to stress. J Biol Chem 1994; 269: 20067–20074.
SionovRV, Haupt Y. The cellular response to p53: The decision between life and death. Oncogene 1999; 18: 6145–6157.
Yeung MC, Lau AS. Tumor suppresor p53 as a component of the tumor necrosis factor-induced, protein kinase PKR-mediated apoptotic pathway in human promonocytic U937 Cells. J Biol Chem 1998; 273: 25198–25202.
Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-®-induced apoptosis by NF-·B. Science1996; 274: 787–789.
Wang C, MayoMW, Baldwin AS Jr. TNF-and cancer therapyinduced apoptosis: Potentiation by inhibition of NF-B. Science1996; 274: 784–787.
Tanaka N, Ishihara M, Kitagawa M, et al. Cellular commitment to oncogene-induced transformation or apoptosis is dependent of the transcription factor IRF-1. Cell 1994; 77: 829–839.
Harada H, Kitagawa M, Tanaka N, et al. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and-2. Science 1993; 259: 971–974.
Kirchhoff S, Koromilas AE, Schaper F, Grashoff M, Sonenberg N, Hauser H. IRF-1 induced cell growth inhibition and interferon induction requires the activity of the protein kinase PKR. Oncogene 1995; 11: 439–445.
Nicholson D, Thornberry N. Caspases: Killer proteases. TIBS1997; 22: 299–306.
Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science 1998; 281: 1305–1308.
Lee SB, Rodriguez D, Rodriguez JR, Esteban M. The apoptosis pathway triggered by the IFN-induced dsRNA activated protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2 and involves ICE-like proteases. Virology 1994; 231: 81–88.
Brun A, Rivas C, Esteban M, Escribano JM, Alonso C. African Swine Fever Virus gene A179L, a viral homologue of bcl-2 protects cells from programmed cell death. Virology 1996; 225: 227–230.
Balachandran S, Kim CN, YehW, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J 1998; 17: 6888–6902.
Donzé O, Dostie J, Sonenberg N. Regulatable expression of the Interferon-induced double-stranded RNA dependent protein kinase PKR induces apoptosis and Fas receptor expression. Virology 1999; 256: 322–329.
Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATPdependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91: 479–489.
Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 1992; 68: 585–596.
Jagus R, Joshi B, Barber GN. PKR, apoptosis and cancer. Int J Biochem Cell Biol 1999; 31: 123–138.
Pavon M, Esteban M. Identification by two-dimensional gel electrophoresis of vaccinia virus and cellular phosphoproteins modified after inducible expression of the dsRNA-activated protein kinase. J Interferon Cytokine Res 1999; 19: 589–599.
De Haro C, Mendez R, Santoyo J. The eIF-2® kinases and the control of protein synthesis. FASE B J 1996; 10: 1378–1387.