Abstract
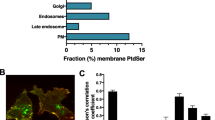
Exposure of the aminophospholipid phosphatidylserine at the outer leaflet of the plasma membrane by apoptotic cells can trigger phagocytic removal of these dying cells. This functionality of phosphatidylserine exposure in the process of phagocytosis is indicated by in vitro studies of mammalian and insect phagocytes. We have studied the in vivo distribution of cell-surface exposed phosphatidylserine by injecting biotinylated Annexin V, a Ca 2+ -dependent phosphatidyl-serine binding protein, into viable mouse and chick embryos and Drosophila pupae. The apparent binding of Annexin V to cells with a morphology which is characteristicof apoptosis and which was present in regions of developmental cell death indicates that phosphatidylserine exposure by apoptotic cells is a phylogenetically conserved mechanism.
Similar content being viewed by others
References
Wyllie A, Kerr J, Currie A. Cell death: The significance of apoptosis. Int Rev Cytol 1980; 68: 251-306.
Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today 1993; vn 14: 131-136.
Ratner S, Schroit AJ, Vinson SB, Fidler IJ. Analogous recognition of phospholipids by insect phagocytes and mammalian macrophages. Proc Soc Exp Biol Med 1986; vn 182: 272-276.
van den Eijnde SM, Boshart L, Reutelingsperger CPM, de Zeeuw CI, Vermeij-Keers C. Phosphatidylserine plasma membrane asymmetry in vivo: a pancellular phenomenon which alters during apoptosis. Cell Death Diff 1997; 4: 311-317.
Reutelingsperger CPM, Hornstra G, Hemker HC. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur J Biochem 1985; vn 151: 625-629.
Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88: 49-92.
Bainbridge SP, Bownes M. Staging the metamorphosis of Drosphila melanogaster. J Embryol Exp Morphol 1981; vn 66: 57-80.
Glücksmann A. Cell deaths in normal vertebrate ontogeny. Biol Rev Camb Philos Soc 1951; 26: 59-86.
Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosphila. Development 1991; 113: 825-839.
Steller H. Mechanisms and genes of cellular suicide. Science 1995; 267: 1445-1462.
Collins MK, Perkins GR, Rodriguez-Tarduchy G, Nieto MA, Lopez-Rivas A. Growth factors as survival factors: regulation of apoptosis. Bioessays 1994; 16: 133-138.
Caelles C, Helmberg A, Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 1994; 370: 220-223.
Falcieri E, Gobbi P, Cataldi A, Zamai L, Faenza I, Vitale M. Nuclear pores in the apoptotic cells. Histoch J 1994; vn 26: 754-763.
Oberhammer FA, Hochegger K, Froschl G, Tiefenbacher R, Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol 1994; vn 126: 827-837.
Zhang C, Ao Z, Seth A, Schlossman SF. A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J Immunol 1996; 157: 3980-3987.
Rotello RC, Fernandez P-A, Yuan J. Anti-apogens and anti-engulfens: monoclonal antibodies reveal specific antigens on apoptotic and engulfment cells during chicken embryo development. Development 1994; 120: 1421-1431.
Fadok VA, Laszlo DJ, Noble PW, Weinstein L, Riches DWH, Henson PM. Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells. J Immunol 1993; 151: 4274-4285.
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 1992; 148: 2207-2216.
Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by sertoli cells of the rat. J Biol Chem 1997; 272: 2354-2358.
Bennet MR, Gibson DF, Schwartz SM, Tait JF. Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ Res 1995; 77: 1136-1142.
Higgins CF. Flip-flop: the transmembrane translocation of lipids. Cell 1994; 79: 393-395.
Diaz C, Schroit AJ. Role of translocases in the generation of phosphatidylserine asymmetry. J Memb Biol 1996; vn 151: 1-9.
Tang XJ, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 1996; 272: 1495-1497.
Martin OC, Pagano RE. Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells. Evidence for a protein-mediated and ATP-dependent process(es). J Biol Chem 1987; 262: 5890-5898.
Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci USA 1984; 81: 3751-3755.
Zachowski A, Henry J-P, Devaux PF. Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature 1989; 340: 75-76.
Williamson P, Bevers EM, Smeets EF, Comfurius P, Schlegel RA, Zwaal RFA. Continuous analysis of the mechanism of activated transbilayer lipid movement in platelets. Biochem 1995; 34: 10448-10455.
Connor J, Pak CH, Schroit AJ. Exposure of phosphatidylserine in the outer leaflet of human red cells. Relationship to cell density, cell age and clearance by mononuclear cells. J Biol Chem 1994; 269: 2399-2404.
Basse F, Stout JG, Sims PJ, Wiedmer T. Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem 1996; 271: 17205-17210.
Homburg CHE, de Haas M, von dem Borne AEGK, Verhoeven AJ, Reutelingsperger CPM, Roos D. Human neutrophils lose their surface FcγRIII and acquire Annexin V binding sites during apoptosis in vitro. Blood 1995; 85: 532-540.
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger CPM. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Meth 1995; 184: 39-51.
van Engeland M, Ramaekers FCS, Schutte B, Reutelingsperger CPM. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996; 24: 131-139.
Koopman G, Reutelingsperger CPM, Kuijten GAM, Keehnen RMJ, Pals ST, van Oers MHJ. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994; 84: 1415-1420.
van den Eijnde SM, Luijsterburg AJM, Boshart L, de Zeeuw CI, Reutelingsperger CPM, Vermeij-Keers C. In situ detection of apoptosis during embryogenesis with Annexin V: from whole mount to ultrastructure. Cytometry 1997; 29: (in press).
Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader J, van Schie RCAA, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and abl. J Exp Med 1995; 182: 1545-1556.
Kaufmann MH. The primitive gut. In: Kaufmann MH, ed. The Atlas of Mouse Development. London: Academic Press 1992: 121-128.
McEvoy L, Williamson P, Schlegel RA. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Natl Acad Sci USA 1986; 83: 3311-3315.
Allen TM, Williamson P, Schlegel RA. Phosphatidylserine as a determinant of reticuloendothelial recognition of liposome models of the erythrocyte surface. Proc Natl Acad Sci USA 1988; 85: 8067-8071.
Schroit AJ, Madsen JW, Tanaka Y. In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J Biol Chem 1985; 260: 5131-5138.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van den Eijnde, S.M., Boshart, L., Baehrecke, E.H. et al. Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis 3, 9–16 (1998). https://doi.org/10.1023/A:1009650917818
Issue Date:
DOI: https://doi.org/10.1023/A:1009650917818