Abstract
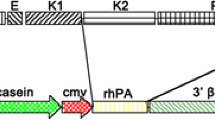
The large scale production of recombinant collagen for use in biomaterials requires an efficient expression system capable of processing a large (>400 Kd) multisubunit protein requiring post-translational modifications. To investigate whether the mammary gland of transgenic animals fulfills these requirements, transgenic mice were generated containing the αS1-casein mammary gland-specific promoter operatively linked to 37 Kb of the human α1(I) procollagen structural gene and 3′ flanking region. The frequency of transgenic lines established was 12%. High levels of soluble triple helical homotrimeric [(α1)3] type I procollagen were detected (up to 8 mg/ml) exclusively in the milk of six out of 9 lines of lactating transgenic mice. The transgene-derived human procollagen chains underwent efficient assembly into a triple helical structure. Although proline or lysine hydroxylation has never been described for any milk protein, procollagen was detected with these post-translational modifications. The procollagen was stable in mil; minimal degradation was observed. These results show that the mammary gland is capable of expressing a large procollagen gene construct, efficiently assembling the individual polypeptide chains into a stable triple helix, and secreting the intact molecule into the milk.
Similar content being viewed by others
References
Barash I, Faerman A, Ratovitsky T, Puzis R, Nathan M, Hurwitz DR and Shani M (1994) Ectopic expression of b-lactoglobulin/human serum albumin fusion genes in transgenic mice: hormonal regulation and in situ localization. Transgenic Res 3: 141–151.
Barsh GS, Roush CL and Gelinas RE (1984) DNA and chromatin structure of the human alpha 1(I) collagen gene. J Biol Chem 259: 14906–14913.
Berg RA and Prockop DJ (1973a) Thermal transition of a nonhydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun 52: 115–120.
Berg RA and Prockop DJ (1973b) Purification of carbon-14 proto collagen and its hydroxylation by prolyl hydroxylase. Biochem 12: 3395–3401.
Brem G, Hartl P, Besenfelder U, Wolf E, Zinovieva N and Pfaller R (1994) Expression of synthetic cDNA sequences encoding human insulin-like growth factor-1 (IGF-1) in the mammary gland of transgenic rabbits. Gene 149: 351–355.
Bruckner P and Prockop DJ (1981) Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Analyt Biochem 110: 360–368.
Buhler T, Bruyere T, Went DF, Stranzinger G and Burki K (1990) Rabbit beta-casein promoter directs secretion of human interleukin-2 into the milk of transgenic rabbits. Bio/Technology 8: 140–143.
Byers PH (1990) Brittle bones-fragile molecules: disorders of collagen gene structure and expression. Trends Genet 6: 293–300.
Cho M-I and Garant PR (1981) Sequential events in the formation of collagen secretion granules with special reference to the development of segment-long spacing-like aggregates. Anat Rec 199: 309–320.
Chomczynski P and Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Analyt Biochem 162: 156–159.
Chu M-L, de Wet WJ, Bernard M and Ramirez F (1985) Fine structure analysis of the human pro-alpha-1(I) collagen gene: promoter structure, Alu repeats, and polymorphic transcripts. J Biol Chem 260: 2315–2330.
Crouch E and Bornstein P (1978) Collagen synthesis by human amniotic fluid cells in culture: Characterization of a procollagen with three identical proa1(I) chains. Biochem 17: 3499–3510.
D'Alessio M, Bernard M, Pretorius PJ, De Wet WJ and Ramirez F (1988) Complete nucleotide sequence of the region encompassing the first twenty-five exons of the human pro-alpha 1(I) collagen gene (COL1A1). Gene 67: 110.
Deak SB, Nicholls A, Pope FM, and Prockop DJ (1983) The molecular defect in a nonlethal variant of osteogenesis imperfecta. Synthesis of proa2(I) chains which are not incorporated into trimers of type I procollagen. J Biol Chem 258: 1192–15197.
Deak SB, van der Rest M and Prockop DJ (1985) Altered helical structure of a homotrimer of a1(I) chains synthesized by fibroblasts from a variant of osteogenesis imperfecta. Collagen Rel Res 5: 305–313.
Dickson LA, Pihlajaniemi T, Deak S, Pope MF, Nicholls AC and Prockop DJ (1984) Nuclease S1 mapping of a homozygous mutation in the carboxyl-propeptide-coding region of the pro alpha 1(I) collagen gene in a patient with osteogenesis imperfecta. Proc Natl Acad Sci USA 81: 4524–4528.
Drews R, Paleyanda RK, Lee TK, Chang RR, Rehemtulla A, Kaufman RJ, DrohanWN and Lubon H (1995) Proteolytic maturation of protein C upon engineering the mouse mammary gland to express furin. Proc Natl Acad Sci USA 92: 10462–10466.
Drohan WN, Zhang D-W, Paleyanda RK, Chang R, Wroble M, Velander WH and Lubon H (1994) Inefficient processing of human protein C in the mouse mammary gland. Transgenic Res 3: 355–364.
Ebert KM, Selgrath JP, DiTullio P, Denman J, Smith TE, Memon MA, Schindler JE, Monastersky GM, Vitale JA and Gordon K (1991) Transgenic production of a variant of human tissue-type plasminogen activator in goat milk: generation of transgenic goats and analysis of expression. Bio/Technology 9: 835–838.
Ebert KM, DiTullio P, Barry CA, Schindler JE, Ayres SL, Smith TE, Pellerin LJ, Meade HM, Denman J and Roberts B (1994) Induction of human tissue plasminogen activator in the mammary gland of transgenic goats. Bio/Technology 12: 699–702.
Echelard Y (1996) Recombinant protein production in transgenic animals. Curr Opin Biotech 7: 536–540.
Elson ML (1993) Dermal filler materials. Dermatologic Clinics 11: 361–367.
Feick RG and Shiozawa JA (1990) A high-yield method for the isolation of hydrophobic proteins and peptides from polyacrylamide gels for protein sequencing. Analyt Biochem 187: 205–211.
Fertala A, Sieron AL, Ganguly A, Li SW, Ala-Kokko L, Anumula KR and Prockop DJ (1994) Synthesis of recombinant human procollagen II in a stably transfected tumour cell line (HT1080). Biochem J 298: 31–37.
Fichard A, Tillet E, Delacoux F, Garrone R and Ruggiero F (1997) Human recombinant a1(V) collagen chain. J Biol Chem 272: 30083–30087.
Fisher LW, Lindner W, Young MF and Termine JD (1989) Synthetic peptide antisera: their production and use in the cloning of matrix proteins. Conn Tissue Res 21: 43–50.
Geddis AE and Prockop DJ (1993) Expression of human COL1A1 gene in stably transfected HT 1080 cells. Matrix 13: 399–405.
Gelman RA, Williams BR and Piez KA (1979) Collagen fibril formation. Evidence for a multistep process. J Biol Chem 254: 180–186.
Ghersi G, Fiura AM and Minafra S (1989) Direct adhesion to type I and homotrimer collagens by breast carcinoma and embryonic epithelial cells in culture: a comparative study. Eur J Cell Biol 50: 279–284.
Gordon K, Lee E, Vitale JA, Smith AE, Westphal H and Hennighausen L (1987) Production of human tissue plasminogen activator in transgenic mice. Bio/Technology 5: 1183–1187.
Gorham SD (1991) Collagen as a biomaterial. In: Byrom D (ed.) Biomaterials, (pp. 55–122) Stockton Press, New York.
Greenberg NM, Anderson JW, Hsueh AJW, Nishimori K, Reeves JJ, de Avila DM, Ward DN and Rosen JM (1991) Expression of biologically active heterodimeric bovine follicle-stimulating hormone in milk of transgenic mice. Proc Natl Acad Sci USA 88: 8327–8331.
Hansson L, Edlund M, Edlund A, Johansson T, Marklund SL, Fromm S, Stronqvist M and Tornell J (1994) Expression and characterization of biologically active human extracellular superoxide dismutase in milk of transgenic mice. J Biol Chem 269: 5358–5363.
Haralson MA, Jacobson HR and Hoover RL (1987) Collagen polymorphism in cultured rat kidney mesangial cells. Lab Invest 57: 513–523.
Hennighausen L (1990) The mammary gland as a bioreactor: production of foreign proteins in milk. Prot Expr Pur 1: 3–5.
Holmes DF, Chapman JA, Prockop DJ and Kadler KE (1992) Growing tips of type I collagen fibrils formed in vitro are near-paraboloidal in shape, implying a reciprocal relationship between accretion and diameter. Proc Natl Acad Sci USA 89: 9855–9859.
Houdebine LM (1994) Production of pharmaceutical proteins from transgenic animals. J Biotechnol 34: 269–287.
Houdebine LM (1995) The production of pharmaceutical proteins from the milk of transgenic animals. Reprod Nutr Dev 35: 609–617.
Hulmes DJS, Bruns RR and Gross J (1983) On the state of aggregation of newly secreted procollagen. Proc Natl Acad Sci USA 80: 388–392.
James DC, Freedman RB, Hoare M, Ogonah OW, Rooney BC, Larionov OA, Dobrovolsky VN, Lagutin OV and Jenkins N (1995) N-glycosylation of recombinant human interferon-g produced in different animal expression systems. Bio/Technology 13: 592–596.
Jimenez S, Harsch M and Rosenbloom J (1973) Hydroxyproline stabilizes the triple helix of chick tendon collagen. Biochem Biophys Res Commun 52: 106–114.
Jimenez SA, Bashey RI, Benditt M and Yankowski R (1977) Identi-fication of collagen a1(I) trimer in embryonic chick tendons and calvaria. Biochem Biophys Res Commun 78: 1354–1361.
John CA, Watson R, Kind AJ, Scott AR, Kadler KE and Bulleid NJ (1999) Expression of an engineered form of recombinant procollagen in mouse milk. Nature Biotechnol 17: 385–389.
Kadler DE, Hojima Y and Prockop DJ (1987) Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem 262: 15696–15701.
Kadler KL (1994) Extracellular matrix I. Fibril forming collagens. In: Protein Profile 1: (pp. 519–638) Academic Press, New York.
Kao WWY, Prockop DJ and Berg RA (1979) Kinetics for the secretion of nonhelical procollagen by freshly isolated tendon cells. J Biol Chem 254: 2234–2243.
Kay EP (1986) Rabbit corneal endothelial cells modulated by polymorphonuclear leukocytes are fibroblasts. Invest Ophthalmol Vis Sci 27: 891–897.
Keefe J, Wauk L, Chu S and DeLustro F (1992) Clinical use of injectable bovine collagen; a decade of experience. Clinical Materials 9: 155–162.
Kivirikko KI, Halaakoski T, Tasanen K, Vuori K, Myllyla R, Parkkonen T and Pihlajaniemi T (1990) Molecular biology of prolyl 4-hydroxylase. Annal NY Acad Sci 580: 132–142.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature 227: 680–685.
Lamberg A, Helaakoski T, Myllyharju J, Peltonen S, Notbolm H, Pihlajaniemi T and Kivirikko KI (1996) Characterization of human type III collagen expressed in a baculovirus system. J Biol Chem 271: 11988–11995.
Leheup BP, Federspiel SJ, Guerry-Force ML, Wetherall NT, Commers PA, DiMari SJ and Haralson MA (1989) Extracellular matrix biosynthesis by cultured fetal rat lung epithelial cells. Lab Invest 60: 791–807.
Lubon H, Paleyanda RK, Velander WH and Drohan WN (1996) Blood proteins from transgenic animal bioreactors. Transfusion Med Rev 10: 131–143.
Massoud M, Attal J, Thepot D, Pointu H, Stinnakre MG, Theron MC, Lopez C and Houdebine LM (1996) The deleterious effects of human erythropoietin gene driven by the rabbit whey acidic protein gene promoter in transgenic rabbits. Reprod Nutr Develop 36: 555–563.
Meade H, Gates L, Lacy E and Lonberg N (1990) Bovine alpha S1-casein gene sequences direct high level expression of active human urokinase in mouse milk. Bio/Technology 8: 443–446.
Miller EJ and Gay S (1982) Collagen: an overview. Methods Enzymol 82: 3–32.
Miller EJ and Rhodes RK (1982) Preparation and characterization of the different types of collagen. ibid 82: 33–64.
Minafra S, Morello V, Glorioso F, La Fiura AM, Tomasino RM, Feo S, McIntosh D and Woolley DE (1989) A new cell line (8701-BC) from primary ductal infiltrating carcinoma of human breast. Br J Cancer 60: 185–192.
Miyata T, Taira T and Noishiki Y (1992) Collagen engineering for biomaterial use. Clin Materials 9: 139–148.
Munksgaard EC, Rhodes M, Mayne R and Butler WT (1978) Collagen synthesis and secretion by rat incisor odontoblasts in organ culture. Eur J Biochem 82: 609–617.
Myllyharju J, Lamberg A, Notbohm H, Fietzek PP, Pihlajaniemi T and Kivirikko KI (1997) Expression of wild-type and modi-fied proalpha chains of human type I procollagen in insect cells leads to the formation of stable [alpha1(I)]2alpha2(I) collagen heterotrimers and [alphal1(I)]3 homotrimers but not [alpha2(I)]3 homotrimers. J Biol Chem 272: 21824–21830.
Narayanan AS and Page RC (1976) Biochemical characterization of collagens synthesized by fibroblasts derived from normal and diseased human gingiva. J Biol Chem 251: 5464–5471.
Nicholls AC, Pope FM and Schloon H (1979) Biochemical heterogeneity of osteogenesis imperfecta. Lancet 1: 1193.
Niemann H, Halter R, Espanion G, Wrenzycki C, Herrmann D, Lemme E, Carnwath JW and Paul D (1996) Expression of human blood clotting factor VIII (FVIII) constructs in the mammary gland of transgenic mice and sheep. J Anim Breeding Genet 113: 437–444.
Paleyanda RK, Zhang D-W, Hennighausen L, McKnight RA and Lubon H (1994) Regulation of human protein C gene expression by the mouse WAP promoter. Transgenic Res 3: 335–343.
Paleyanda RK, VelanderWH, Lee TK, Scandella DH, Gwazdauskas FC, Knight JW, Hoyer LW, Drohan WN and Lubon H (1997) Transgenic pigs produce functional human factor VIII in milk. Nature Biotechnol 15: 971-975.
Parker MI, Smith AA and Gevers W (1989) Absence of alpha 2(1) procollagen synthesis in a clone of SV40-transformed WI-38 human fibroblasts. J Biol Chem 264: 7147–7152.
Pihlajaniemi T, Dickson LA, Pope FM, Korhonen VR, Nicholls A, Prockop DJ and Myers JC (1984) Osteogenesis imperfecta. Cloning of a proa2(I) collagen gene with a frame-shift mutation. J Biol Chem 259: 12941–12944.
Pittius CW, Hennighausen L, Lee E, Westphal H, Nicols E, Vitale J and Gordon K (1988) A milk protein gene promoter directs the expression of human tissue plasminogen activator cDNA to the mammary gland in transgenic mice. Proc Natl Acad Sci USA 85: 5874–5878.
Platenburg GJ, Kootwijk EP, Kooiman PM, Woloshuk SL, Nuijens JH, Krimpenfort PJ, Pieper FR, de Boer HA and Strijker J (1994) Expression of human lactoferrin in milk of transgenic mice. Transgenic Res 3: 99–108.
Prockop DJ and Kivirikko KI (1995) Collagens: Molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64: 403–434.
Prunkard D, Cottingham I, Garner I, Bruce S, Dalrymple M, Lasser G, Bishop P and Foster D (1996) High level expression of recombinant human fibrinogen in the milk of transgenic mice. Nature Biotechnol: 14, 867–871.
Rokkones E, Fromm SH, Kareem BN, Klungland, Olstad OK, Hogset A, Iversen J, Bjoro K and Gautvik KM (1995) Human parathyroid hormone as a secretory peptide in milk of transgenic mice. J Cell Biochem 59A: 168–176.
Rupard JH, Dimari SJ, Damjanov I and Haralson MA (1988) Synthesis of type I homotrimer collagen molecules by cultured human lung adenocarcinoma cells. Am J Pathol 133: 316–326.
Sandell LJ and Boyd CD (1990) Conserved and divergent sequence and functional elements within collagen genes. In Sandell LJ and Boyd CD (eds), Extracellular Matrix Genes (pp. 1–56) Academic Press, New York.
Saski T, Katsuhiko A, Ono M, Yamaguchi T, Furuta S and Nagai Y (1987) Ehlers-Danlos syndrome. A variant characterized by deficiency of proa2 chain of type I procollagen. Arch Dermatol 123: 76–79.
Shani M, Barash I, Nathan M, Ricca G, Searfoss GH, Dekel I, Faerman A, Givol D and Hurwitz DR (1992) Expression of human serum albumin in the milk of transgenic mice. Transgenic Res 1: 195–208.
Stromqvist M, Tornell J, Edlund M, Edlund A, Johansson T, Lindgren K, Lundberg L and Hansson L (1996) Recombinant human bile salt-stimulated lipase: an example of defective O-glycosylation of a protein produced in milk of transgenic mice. Transgenic Res 5: 475–485.
Subramanian A, Paleyanda RK, Lubon H, Williams BL, Gwazdauskas FC, Knight JW, Drohan WN and Velander WH (1996) Rate limitations in post-translational processing by the mammary gland of transgenic animals. Annal NY Acad Sci 782: 87–96.
Thepot D, Devinoy E, Fontaine M-L, Stinnakre M-G, Massoud M, Kann G and Houdebine ML (1995) Rabbit whey acid protein gene upstream region controls high-level expression of bovine growth hormone in the mammary gland of transgenic mice. Mol Reprod Develop 42: 261–267.
Towbin H and Staehelin T (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA 76: 4350-4354.
Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA and Weiner HL (1993) Effects of oral administration of type II collagen on rheumatoid arthritis. Science 261: 1727–1730.
Tromp G, Kuivaniemi H, Stacey A, Shikata H, Baldwin CT, Jaenish R and Prockop DJ (1988) Structure of a full-length cDNA clone for the prepro alpha 1(I) chain of human type I procollagen. Biochem J 253: 919–922.
Uitto J (1979) Collagen polymorphism: Isolation and partial characterization of a1(I) trimer molecules in normal human skin. Arch Biochem Biophys 192: 371–379.
Uusi-Oukari M, Hyttinen J-M, Korhonen V-P, Vasti A, Alhonen L, Janne OA and Janne J (1997) Bovine aS1-casein gene sequences direct high level expression of human granulocyte-macrophage colony-stimulating factor in the milk of transgenic mice. Transgenic Res 6: 75–84.
Van Der Rest M and Bruckner P (1993) Collagen's diversity at the molecular and supramolecular levels. Curr Opin Structural Biol 3: 430–436.
Velander WH, Johnson JL, Page RL, Russell CG, Subramanian A and Wilkins TD (1992) High-level expression of a heterologous protein in the milk of transgenic swine using the cDNA encoding human protein C. Proc Natl Acad Sci USA 89: 12003–12007.
Vuorela A, Myllyharju J, Nissi R, Pihlajaniemi T and Kivirikko KI (1997) Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast Pichia pastoris. EMBO J 16: 6702–6712.
Vuorio E and de Crombrugghe B (1990) The family of collagen genes. Annu Rev Biochem 59: 837–872.
Wall RJ, Pursel VG, Shamay A, McKnight RA, Pittius CW and Hennighausen L (1991) High-level synthesis of a heterologous milk protein in the mammary glands of transgenic swine. Proc Natl Acad Sci USA 88: 1696–1700.
Wallace D and Thompson A (1983) Description of collagen fibril formation by a theory of polymer crystallization. Biopolymers 25: 1793–1811.
Wei Y, Yarus S, Greenberg NM, Whitsett J and Rosen JM (1995) Production of human surfactant protein C in milk of transgenic mice. Transgenic Res 4: 232–240.
Wen J, Kawamata Y, Tojo H, Tanaka, S and Tachi C (1995) Expression of whey acid protein (WAP) genes in tissues other than the mammary gland in normal and transgenic mice expressing mWAP/hGH fusion gene. Mol Reprod Develop 41: 399–406.
Wright G, Carver A, Cottom D, Reeves D, Scott A, Simons P, Wilmut I, Garner I and Coleman A (1991) High level expression of active human alpha-1-antitrypsin in the milk of transgenic sheep. Bio/Technology 9: 830–834.
Yarus S, Greenberg NM, Wei Y, Whitsett JA, Weaver TE and Rosen JM (1997) Secretion of unprocessed human surfactant protein B in milk of transgenic mice. Transgenic Res 6: 51–57.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toman, P.D., Pieper, F., Sakai, N. et al. Production of recombinant human type I procollagen homotrimer in the mammary gland of transgenic mice. Transgenic Res 8, 415–427 (1999). https://doi.org/10.1023/A:1008959924856
Issue Date:
DOI: https://doi.org/10.1023/A:1008959924856