Abstract
We previously described an expression cassette that relies on the tobacco etch virus (TEV) nuclear inclusion a (NIa) protease and leads to the coordinated accumulation of multiple proteins through self processing of a polyprotein [21]. However, low levels of proteins accumulated when the full-length protease was encoded within the polyprotein [22].
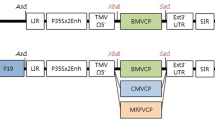
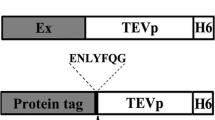
Studies were conducted to evaluate whether the disruption of NIa nuclear localization would affect the levels of proteins produced via the cassette. Modifications comprised either removal of its nuclear localization signals (NLSs), removal of the VPg domain (which includes the NLSs), and fusion to the 6 kDa protein, previously demonstrated to be a viral cytoplasmic anchor [28]. In in vitro translation reactions and in vivo protoplast experiments the modified NIa retained sequence-specific proteolysis. Moreover, the removal of the NLSs correlated with an increase in GUS reporter accumulation. The modified cassette, pPRO10, led to the synthesis of up to three viral coat protein (CPs) in addition to NIa. However, the accumulation of proteins in protoplasts depended upon the position of the CP coding sequence within the cassette as well as on the stability of the protein.
Similar content being viewed by others
References
Baratova LA, Grebenshchikov NI, Shishkov AV, Kashirin IA, Radavsky JL, Jarvelkulg L, Saarma M: The topography of the surface of potato virus X: tritium planigraphy and immunological analysis. J Gen Virol 73: 229–235 (1992).
Barker H, Reavy B, Kumar A, Webster KD, Mayo MA: Restricted viruses multiplication in potatoes transformed with the coat protein gene of potato leafroll luteovirus: similarities with a type of host gene-mediated resistance. Ann Appl Biol 120: 55–64 (1992).
Bravo-Almonacid F: Desarrollo de métodos de diagnóstico y de plantas trangénicas de papa para el control de infecciones producidas por el virus PVY. Universidad Nacional de La Plata (1992).
Carrington JC, Dougherty WC: A viral cleavage site cassette: Identification of amino acid sequences required for tobacco etch virus polyprotein processing. Proc Natl Acad Sci USA 85: 3391–3395 (1988).
Carrington JC, Dougherty WG: Processing of the Tobacco Etch virus 49K protease requires autoproteolysis. Virology 160: 355–362 (1987).
Carrington JC, Freed DD, Leinicke AJ: Bipartite signal sequence mediates nuclear translocation of the plant potyviral NIa protein. Plant Cell 3: 953–962 (1991).
Carrington JC, Freed DD, Oh CS: Expression of potyviral polyproteins in transgenic plants reveals three proteolytic activities required for complete processing. EMBO J 9: 1347–1354 (1990).
Carrington JC, Haldeman R, Dolja VV, Restrepo-Hartwig MA: Internal cleavage and transproteolytic activities of the VPgproteinase (NIa) of tobacco etch potyvirus in vivo. J Virol 67: 6995–7000 (1993).
Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC: Green fluorescent protein as a marker for gene expression. Science 263: 802–805 (1994).
del Vas M: Obtención y caracterización de plantas de interés agropecuario. Universidad de Buenos Aires-Argentina (1993).
Dougherty WG, Carrington JC, Cary SM, Parks TD: Biochemical and mutational analysis of a plant virus polyprotein cleavage site. EMBO J 7: 1281–1288 (1988).
Dougherty WG, Cary SM, Parks TD: Molecular genetic analysis of a plant virus polyprotein cleavage site: a model. Virology 171: 356–364 (1989).
Dougherty WG, Parks TD: Molecular genetic and biochemical evidence for the involvement of the heptapeptide cleavage sequence in determining the reaction profile at two tobacco etch virus cleavage sites in cell-free assays. Virology 172: 145–155 (1989).
Dougherty WG, Parks TD: Post-translational processing of the tobacco etch virus 49-kDa small nuclear inclusion polyprotein: identification of an internal cleavage site and delimitation of VPg and proteinase domains. Virology 183: 449–456 (1991).
Heinlein M, Epel B, Padgett HS, Beachy RN: Interaction of tobamovirus movement proteins with plant cytoskeleton. Science 270: 1983–1985 (1995).
Jefferson RA, Wilson KJ: The GUS gene fusion system. In: Gelvin SB, Schilperoort RA (eds) Plant Molecular Biology Manual, 1–33. Kluwer Academic Publishers, Dordrecht, Netherlands (1991).
Kawchuk LM, Martin RR, McPherson J: Sense and antisense RNA-mediated resistance to potato leafroll virus in Russet Burbank potato plants. Mol Plant-Microbe Interact 4: 247–253 (1991).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680–685 (1970).
Lawson C, Kaniewski W, Haley L, Rozman R, Newell C, Sanders P, Turner N: Engineering resistance to mixed virus infection in a commercial potato cultivar: resistance to PVY in transgenic Russet Burbank. Bio/technology 8: 127–134 (1990).
Logemann J, Schell J, Willmitzer L: Improved method for the isolation of RNAfrom plant tissues. Anal Biochem 163: 16–20 (1987).
Marcos JF, Beachy RN: In vitro characterization of a cassette to accumulate multiple proteins through synthesis of a selfprocessing polypeptide. Plant Mol Biol 24: 495–503 (1994).
Marcos JF, Beachy RN: Transgenic accumulation of two plant virus coat protein genes on a single self-processing polypeptide. J Gen Virol 78: 1771–1778 (1997).
Orman BE, Celnik RM, Mandel AM, Torres HN, Mentaberry AN: Complete complementary DNA sequence of a South American isolate of potato virus X. Virus Res 16: 293–306 (1990).
Padgett HS, Beachy RN: Analysis of a tobacco mosaic virus strain capable of overcoming N gene-mediated resistance. Plant Cell 5: 577–586 (1993).
Park YD, Papp I, Moscone EA, Matzke AJM, Matzke MA: Gene silencing mediated by promoter homology occurs at the level of transcription and results in meiotically heritable alterations in methylation and gene activity. Plant J 9: 183–194 (1996).
Parks TD, Howard ED, Wolpert TJ, Arp DJ, Dougherty WG: Expression and purification of a recombinant tobacco etch virus NIa proteinase: biochemical analyses of the full-length and a naturally occurring truncated proteinase form. Virology 210: 194–201 (1995).
Restrepo MA, Freed DD, Carrington JC: Nuclear transport of plant potyviral proteins. Plant Cell 2: 987–998 (1990).
Restrepo-Hartwig MA, Carrington JC: The tobacco etch potyvirus 6-kilodalton protein is membrane associated and involved in viral replication. J Virol 68: 2388–2397 (1994).
Sambrook J, Fritsch E, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
von Bodman BS, Domier L, Farrand SK: Expression of multiple eukaryotic genes from a single promoter in Nicotiana. Bio/technology 13: 587–591 (1995).
Watanabe Y, Emori Y, Ooshika I, Meshi T, Ohno T, Okada Y: Synthesis of TMV-specificRNAs and proteins at the early stage of infection in tobacco protoplasts: transient expression of the 30K protein and its mRNA. Virology 133: 18–24 (1984).
Watanabe Y, Meshi T, Okada Y: Infection of tobacco protoplasts with in vitro transcribed tobacco mosaic virus RNA using an improved electroporation protocol. FEBS Lett 219: 65–69 (1987).
Watanabe Y, Ohno T, Okada Y: Virus multiplication in tobacco protoplasts inoculated with tobacco mosaic virus encapsulated in large unilamellar vesicle liposomes. Virology 120: 478–480 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ceriani, M.F., Marcos, J.F., Esteban Hopp, H. et al. Simultaneous accumulation of multiple viral coat proteins from a TEV-NIa based expression vector. Plant Mol Biol 36, 239–248 (1998). https://doi.org/10.1023/A:1005952001774
Issue Date:
DOI: https://doi.org/10.1023/A:1005952001774