Abstract
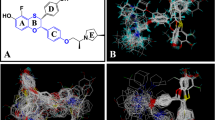
We present the results from a Comparative Molecular Field Analysis (CoMFA) and docking study of a diverse set of 36 estrogen receptor ligands whose relative binding affinities (RBA) with respect to 17β-Estradiol were available in both isoforms of the nuclear estrogen receptors (ERα, ERβ). Initial CoMFA models exhibited a correlation between the experimental relative binding affinities and the molecular steric and electrostatic fields; ERα: r2=0.79, q2=0.44 ERβ: r2=0.93, q2=0.63. Addition of the solvation energy of the isolated ligand improved the predictive nature of the ERβ model initially; r2=0.96, q2=0.70 but upon rescrambling of the data-set and reselecting the training set at random, inclusion of the ligand solvation energy was found to have little effect on the predictive nature of the CoMFA models. The ligands were then docked inside the ligand binding domain (LBD) of both ERα and ERβ utilizing the docking program Gold, after-which the program CScore was used to rank the resulting poses. Inclusion of both the Gold and CScore scoring parameters failed to improve the predictive ability of the original CoMFA models. The subtype selectivity expressed as RBA(ERα/ERβ) of the test sets was predicted using the most predictive CoMFA models, as illustrated by the cross-validated r2. In each case the most selective ligands were ranked correctly illustrating the utility of this method as a prescreening tool in the development of novel estrogen receptor subtype selective ligands.
Similar content being viewed by others
References
Kuiper, G.G.J.M., Enmark, E., Pelto-Huikko, M., Nilsson, S., Gustafsson, J.-A. Proc. Natl. Acad. Sci. USA 93 (1996) 5925.
Mosselman, S., Polman, J., Dijkema, R. FEBS Letters 392 (1996) 49.
Fawell, S.E., Lees, J.A., White, R., Parker, M.G. Cell 60 (1990) 953.
Kuiper, G.G.J.M., Carlsson, B., Grandien, K., Enmark, E., Haggblad, J., Nilsson, S., Gustafsson, J.-A. Endocrinology 138 (1997) 863.
Register, T.C., Adams, M.R. The Journal of Steroid Biochemistry and Molecular Biology 64 (1998) 187.
Paech, K., Webb, P., Kuiper, G.G.J.M., Nilsson, S., Gustafsson, J.-A., Kushner, P.J., Scanlan, T.S. Science 277 (1997) 1508.
Montano, M.M., Jaiswal, A.K., Katzenellenbogen, B.S. J. Biol. Chem. 273 (1998) 25443.
Mortensen, D.S., Rodriguez, A.L., Sun, J., Katzenellenbogen, B.S., Katzenellenbogen, J.A. Bioorganic & Medicinal Chemistry Letters 11 (2001) 2521.
Stauffer, S.R., Coletta, C.J., Tedesco, R., Nishiguchi, G., Carlson, K., Sun, J., Katzenellenbogen, B.S., Katzenellenbogen, J.A. J. Med. Chem. 43 (2000) 4934.
Meyers, M.J., Sun, J., Carlson, K.E., Katzenellenbogen, B.S., Katzenellenbogen, J.A. J. Med. Chem. 42 (1999) 2456.
Gao, H., Williams, C., Labute, P., Bajorath, J. Journal of Chemical Information & Computer Science 39 (1999) 164.
Gao, H., Katzenellenbogen, J. A., Garg, R., Hansch, C. Chem. Rev. 99 (1999) 723.
Cramer, R.D., III, Patterson, D.E., Bunce, J.D. J. Am. Chem. Soc. 110 (1988) 5959.
Tong, W., Perkins, R., Xing, L., Welsh, W.J., Sheehan, D.M. Endocrinology 138 (1997) 4022.
Sadler, B.R., Cho, S.J., Ishaq, K.S., Chae, K., Korach, K.S. J. Med. Chem. 41 (1998) 2261.
Sippl, W. J. Comp.-Aided Mol. Des. 14 (2000) 559.
Sippl, W., Holtje, H.D. Theochem 503 (2000) 31.
Sippl, W. Bioorg. & Med. Chem. 10 (2002) 3741.
Hopfinger, A.J. J. Am. Chem. Soc. 102 (1980) 7196.
Pastor, M., Cruciani, G., McLay, I., Pickett, S., Clementi, S. J. Med. Chem. 43 (2000) 3233.
Charifson, P.S., Corkery, J.J., Murcko, M.A., Walters, W.P. J. Med. Chem. 42 (1999) 5100.
Clark, R.D., Strizhev, A., Leonard, J.M., Blake, J.F., Matthew, J.B. Journal of Molecular Graphics and Modelling 20 (2002) 281.
Vieth, M., Cummins, D.J. J. Med. Chem. 43 (2000) 3020.
Lozano, J.J., Pastor, M., Cruciani, G., Gaedt, K., Centeno, N.B., Gago, F., Sanz, F. Journal of Computer-Aided Molecular Design. 14 (2000) 341.
Brzozowski, A.M., Pike, A.C.W., Dauter, Z., Hubbard, R.E., Bonn, T., Engström, O., Öhman, L., Greene, G.L., Gustafsson, J.-Å., Carlquist, M. Nature 389 (1997) 753.
Pike, A.C.W., Brzozowski, A.M., Hubbard, R.E., Bonn, T., Thorsell, A.-G., Engstrom, O., Ljunggren, J., Gustafsson, J.-A., Carlquist, M. EMBO J. 18 (1999) 4608.
Jones, G., Willet, P., Glen, R.C. Journal of Molecular Biology 245 (1995) 43.
Jones, G., Willett, P., Glen, R.C., Leach, A.R., Taylor, R. Journal of Molecular Biology 267 (1997) 727.
Bissantz, C., Folkers, G., Rognan, D. J. Med. Chem. 43 (2000) 4759.
Åqvist, J., Medina, C., Samuelsson, J.-E. Protein Engineering 7 (1994) 385.
Holloway, M.K., Wai, J.M., Halgren, T.A., Fitzgerald, P.M.D., Vacca, J.P., Dorsey, B.D., Levin, R.B., Thompson, W. J., Chen, L. J., et al. J. Med. Chem. 38 (1995) 305.
Ortiz, A.R., Pisabarro, M.T., Gago, F., Wade, R.C. J. Med. Chem. 38 (1995) 2681.
Pérez, C., Pastor, M., Ortiz, A.R., Gago, F. J. Med. Chem. 41 (1998) 836.
Dhar, A., Liu, S., Klucik, J., Berlin, K.D., Madler, M.M., Lu, S., Ivey, R.T., Zacheis, D., Brown, C.W., Nelson, E.C., Birckbichler, P.J., Benbrook, D. M. Journal ofMedicinal Chemistry. 42 (1999) 3602.
Bertelli, M., El-Bastawissy, E., Knaggs, M.H., Barrett, M.P., Hanau, S., Gilbert, I.H. Journal of Computer-Aided Molecular Design. 15 (2001) 465.
Tripos SYBYL; 6.7 ed.; Tripos Inc.: St Louis, 1995.
Jayatilleke, P.R.N., Nair, A.C., Zauhar, R., Welsh, W. J. J. Med. Chem. 43 (2000) 4446.
Qiu, D., Shenkin, P.S., Hollinger, F.P., Still, W.C. J. Phys. Chem. A 101 (1997) 3005.
Mohamadi, F., Richards, N.G.J., Guida, W.C., Liskamp, R., Lipton, M., Caulfield, C., Chang, G., Hendrickson, T., Still, W.C. J. Comput. Chem. 11 (1990) 440.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wolohan, P., Reichert, D.E. CoMFA and docking study of novel estrogen receptor subtype selective ligands. J Comput Aided Mol Des 17, 313–328 (2003). https://doi.org/10.1023/A:1026104924132
Issue Date:
DOI: https://doi.org/10.1023/A:1026104924132