Abstract
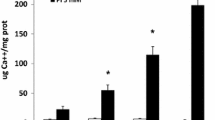
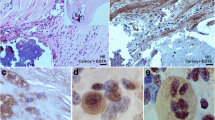
The localization of osteoglycin (OG), one of the corneal keratan sulfate proteoglycans, was studied in different normal rabbit tissues, as well as in atherosclerotic lesions, by means of in situ hybridization and immunohistochemistry. OG was associated with the vasculature of all the organs analyzed. Normal aortas showed abundance of the protein in the adventitia and focally in the media. Peripheral vessels showed OG localized only in the adventitia. OG mRNA was restricted to vascular smooth muscle cells, pericytes, and fibroblasts in aorta and skeletal muscle. In striated muscle, OG was abundant and distributed in foci around muscles and vessels, whereas in visceral muscle, the protein was homogeneously distributed throughout the extracellular matrix. In all the other organs studied, OG was only associated with the vasculature, with the exception of the lung and liver. In these two organs, the protein accumulated also around cartilage, alveoli, and hepatic duct. In atherosclerotic lesions, OG mRNA was down-regulated in the media and up-regulated in the activated endothelium and thick neo-intima, whereas the protein accumulated in the front edge of migrating smooth muscle cells. We conclude that OG is a basic component of the vascular extracellular matrix. OG also plays a role in atherosclerosis, and might be useful for therapeutic interventions. In addition, the possible involvement of OG in maintaining physical properties of tissues is discussed.
Similar content being viewed by others
References
Bentz H, Nathan RM, Rosen DM, Armstrong RM, Thompson AY, Segarini PR, Mathews MC, Dasch JR, Piez KA, Seyedin SM: Purification and characterization of a unique osteoinductive factor from bovine bone. J Biol Chem 264: 20805-20810, 1989
Funderburgh JL, Corpuz LM, Roth MR, Funderburgh ML, Tasheva ES, Conrad GW: Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem 272: 28089-28095, 1997
Blochberger TC, Vergnes J-P, Hempel J, Hassells JR: cDNA to chick Lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J Biol Chem 267: 347-352, 1992
Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV: Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 136: 729-743, 1997
Rada JA, Cornuet PK, Hassell JR: Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res 56: 635-648, 1993
Yamaguchi Y, Mann DM, Ruoslahti E: Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 346: 281-284, 1990
Krusius T, Finne J, Margolis RK, Margolis RU: Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J Biol Chem 261: 8237-8242, 1986
Funderburgh JL, Caterson B, Conrad GW: Distribution of proteoglycans antigenically related to corneal keratan sulfate proteoglycan. J Biol Chem 262: 11634-11640, 1987
Scott JE: Proteoglycan-fibrillar collagen interactions. Biochem J 252: 313-323, 1988
Vogel KG, Paulsson M, Heinegard D: Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycans of tendon. Biochem J 223: 587-597, 1984
Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H: Lumican regulates collagen fibril assembly: Skin fragility and corneal opacity in the absence of lumican. J Cell Biol 14: 1277-1286, 1998
Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW: Molecular cloning and tissue distribution of keratocan. J Biol Chem 271: 9759-9763, 1996
Tasheva ES, Corpuz LM, Funderburgh JL, Conrad GW: Differential splicing and alternative polyadenylation generate multiple mimecan mRNA transcripts J Biol Chem 272: 32551-32556, 1997
Dash JR, Pace DR, Avis PD, Bentz H, Chu S: Characterization of monoclonal antibodies recognizing bovine bone osteoglycin. Conn Tissue Res 30: 11-21, 1993
Shanahan CM, Cary NRB, Osbourn JK, Weissberg PL: Identification of osteoglycin as a component of the vascular matrix. Differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscl Thromb Vascular Biol 17: 2437-2447, 1997
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156-159, 1987
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York, 1989
Thieszen SL, Rosenquist TH: Expression of collagens and decorin during aortic arch artery development: Implications for matrix pattern formation. Matrix Biol 14: 573-582, 1994
Scott JE, Orford CR: Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J 197: 213-216, 1981
Hedbom E, Heinegard D: Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem 268: 27307-27312, 1993.
Schonherr E, Hausser H, Beavan L, Kresse H: Decorin-type I collagen interaction. J Biol Chem 270: 8877-8883, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fernández, B., Kampmann, A., Pipp, F. et al. Osteoglycin expression and localization in rabbit tissues and atherosclerotic plaques. Mol Cell Biochem 246, 3–11 (2003). https://doi.org/10.1023/A:1023487424751
Issue Date:
DOI: https://doi.org/10.1023/A:1023487424751