Abstract
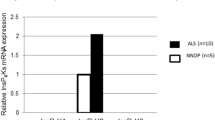
Amyotrophic lateral sclerosis (ALS) is characterized by the selective degeneration of specific populations of cranial and spinal motor neurons. In this study, we examined the expression of the high affinity functional receptor for BDNF, TrkB, and assessed the functional state of TrkB by examining the level of phosphorylation on tyrosine residues in ALS spinal cords. The data showed that TrkB-immunoprecipitates prepared from cell-free lysates of ALS spinal cords by use of an anti-TrkB antibody contained much more TrkB protein than from controls. These TrkB proteins expressed in ALS spinal cords, however, are much less phosphorylated on tyrosine residues than those of controls. Moreover, RT-PCR analysis of TrkB mRNA in ALS spinal cords demonstrated that the expression of Trk B mRNA is also upregulated in ALS spinal cords compared with those of controls. These data strongly suggest that there exists an abnormality in TrkB-mediated intracellular signaling in ALS spinal cords and shed a light on the possibility of the therapeutic intervention by normalizing this intracellular signaling.
Similar content being viewed by others
REFERENCES
Hughes, J. T. 1982. Pathology of amyotrophic lateral screlosis. Pages 61–73, in Rowland, L. P. (eds.), Human Motor Neuron Diseases, Raven Press, New York.
Rosen, D. R., Siddique, T., Patterson, D., Figlewcz, D. A., Sapp, P., Hentai, A., Donaldson, D., Goto, J., O'Regan, J. P., Deng, H.-X., Rahmni, Z., Krizus, A., McKenna-Ysek, D., Cayabyas, A., Gaston, S. M., Berger, R., Tanzi, R., Haperin, J. J., Herzfeld, B., Van den Bergh, R., Hung, W., Bird, T., Deng, G., Mulder, D. W., Smyth, C., Laing, N. G. F., Soriano, E., Pericak-Vance, M. A., Haines, J., Rouleau, G. A., Gusella, J. S., Horvitz, H. R., and Brown, R. H. Jr. 1993. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62.
Siddique, T., Nijhanwan, D., and Hentati, A. 1996. Molecular genetic basis of familial ALS. Neurology 47 (suppl. 2):S27-S35.
Xu, Z., Cork, L. C., Griffin, J. W., and Cleveland, D. W. 1993. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell 73:23–33.
Bowling, A. C., Schulz, J. B., Brown, R. H. Jr., and Beal, M. F. 1993 Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 61:2322–2325.
Rattan, R. R., Murphy, T. H., and Baraban, J. M. 1994. Oxidative stress induces apoptosis in embryonic cortical neurons. J. Neurochem. 62:376–379.
Rothstein, J. D. 1994. Excitotoxicity hypothesis. Neurology 47 (suppl. 2):S19-S26.
Smith, R. G., Siklos, L., Alexianu, M. E., Engelhard, J. I., Mosier, D. R., Colom, L., Mohamed, A. H., and Appel, S. H. 1996. Autoimmunity and ALS. Neurology 47 (suppl. 2):S40-S46.
Elliott, J. L. and Snider, W. D. 1996. Motor neuron growth factors. Neurology 47 (suppl. 2):S47-S53.
Rothstein, J. D., Bristol, L. A., Hosler, B., Brown, R. H. Jr., and Kuncl, R. W. 1994. Chronic inhibition of superoxide dismutase produces apoptotic death of spinal neurons. Proc. Natl. Acad. Sci. U.S.A. 91:4155–4159.
Yan, Q., Elliott, J., and Snider, W. D. 1992. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature 360:753–755.
Sendtner, M., Holtmann, B., Kolbeck, R., Thoenen, H., and Barde, Y.-A. 1992. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 360:757–759.
Koliatsos, V. E., Clatterbuck, R. E., Winslow, J. W., Cayouette, M. H., and Price, D. L. 1992. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 10:359–367.
Barbacid, M. 1995. Neurotropic factors and their receptors. Curr. Opin. Cell Biol. 7:148–155.
Ernfors, P., Lee, K-F., Kucer, J., and Jaenish, R. 1994. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of proprioceptive afferents. Cell 77:503–512.
Klein, R., Silos-Santiago, I., Smeyne, R. J., Lira, S. A., Brambilla, R., Bryant, S., Zhang, L., Snider, W. D., and Barbacid, M. 1994. Disruption of the neurotrophin-3 receptor gene trkC eleimunates Ia muscle afferents and results in abnormal movements. Nature 368:249–251.
Jones, K. R., Farinas, I., Backus, C., and Reichert, F. 1994. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 75:989–999.
Conover, J. C., Erickson, J. T., Katz, D. M., Bianchi, L. M., Poueymirou, W. T., McClain, J., Pan, L., Helgren, M., Ip, N. Y., Boland, P., Friedman, B., Wiegand, S., Vejsada, R., Kato, A. C., DeChiara, T. M., and Yancopoulos, G. D. 1995. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature 375:235–240.
Indo, Y., Tsuruta, M., Hayashido, Y., Karim, M. A., Ohta, K., Kawano, T., Mitsubuchi, H., Tonoki, H., Awaya, Y., and Matsuda, I. 1996. Mutations in the TrkA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature Genet. 13:485–488.
Silos-Santiago, I., Molliver, D. C., Ozaki, S., Smeyne, R. J., Fagan, A. M., Barbacid, M., and Snider, W. D. 1995. Non-TrkA-expressing small DRG neurons are lost in TrkA deficient mice. J. Neurosci. 15:5929–5942.
Klein, R., Nanduri, V., Jing, S. A., Lamballe, F., Tapley, P., Bryant, S., Cordon-Cardo, C., Jones, K. R., Reichardt, L. F., and Barbacid, M. 1991. The TrkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 66:395–403.
Klein, R., Parada, L. F., Coulier, F., and Barbacid, M. 1989. TrkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 8:3701–3718.
Kaplan, D. R., Matsumoto, K., Lucarelli, E., and Thiele, C. J. 1993. Induction of Trk B by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Neuron 60:1540–1547.
Mutoh, T., Tokuda, A., Guroff, G., and Fujiki, N. 1993. The effect of B subunit of cholera toxin on the action of nerve growth factor on PC12 cells. J. Neurochem. 60:1540–1547.
Mutoh, T., Tokuda, A., Miyadai, T., Hamaguchi, M., and Fujiki, N. 1995. The ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. U.S.A. 92:5087–5091.
Mutoh, T., Tokuda, A., Inokuchi, J.-I., and Kuriyama, M. 1998. Glucosylceramide synthase inhibitor inhibits the action of nerve growth factor in PC12 cells. J. Biol. Chem. 273:26001–26007.
Pitto, M., Mutoh, T., Kuriyama, M., Ferarretto, A., Palesteni, P., and Masserini, M. 1998. Influence of endogenous GM1 ganglioside on TrkB activity in cultured cerebellar granule cells. FEBS Lett. 483:93–96.
Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72:248–254.
Yamamoto, M., Sobue, G., Yamamoto, K., Terao, S., and Mitsuma, T. 1996. Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, TrkA, TrkB, and TrkC) in the adult human peripheral nervous system and nonneural tissues. Neurochem. Res. 21:929–938.
Yamamoto, M., Sobue, G., Yamamoto, K., Terao, S., and Mitsuma, T. 1996. Expression of glial cell line-derived growth factor mRNA in the spinal cord and muscle in amyotrophic lateral sclerosis. Neurosci. Lett. 204:117–120.
Iwata, H., Ito, S., Mutoh, T., Ishiguro, H., and Hamaguchi, M. 1994. Abundant but inactive expression of high affinity nerve growth factor receptor (p140proto-trkA) in neuroblastoma patients of good prognosis. Jap. J. Cancer Res. 82:32–39.
Reed, J. C. 1997. Double identity for proteins of the Bcl-2 family. Nature 387:773–776.
Wertz, I. E. and Hanley, M. R. 1996. Diverse molecular provocation of programmed cell death. Trends Biochem. Sci. 21:359–364.
Wyllie, A. H., Kerr, J. F., and Currie, A. R. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251–306.
Pettmann, B. and Henderson, C. E. 1998. Neuronal cell death. Neuron 20:633–647.
Mu, X., He, J., Anderson, D. W., Trojanowski, J. Q., and Springer, J. E. 1996. Altered expression of bcl-2 and bax mRNA in amyotrophic lateral sclerosis spinal cord motor neurons. Ann. Neurol. 40:379–386.
Seeburger, J. L., Tarras, S., Natter, H., and Springer, J. E. 1993. Spinal cord motor neurons express p75NGFR and p145trkB mRNA in amyotrophic lateral sclerosis. Brain Res. 621:111–115.
Oppenheim, R. W., Houenou, L. J., Johnson, J. E., Lin, L. F. H., Li, L., Lo, A. C., Newsome, A. L., Prevette, D. M., and Wang, S. 1995. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature 373:344–346.
Vejsada, R., Sagot, Y., and Kato, A. C. 1995. Quantitative comparison of the transient rescue effects of neurotrophic factors on axotomized motoneurons in vivo. Eur. J. Neurosci. 7:108–115.
Lindvall, O., Kokaia, Z., Bengzon, J., Elmer, E., and Kokaia, M. 1994. Neurotrophins and brain insults. Trends Neurosci. 17:490–496.
Lange, D. J., Felice, K. J., Festoff, B. W., Gawel, M. J., Gelinas, D. F., Kratz, R., Lai, E. C., Murphy, M. F., Natter, H. M., Norris, F. H., Rudnicki, S., and the North American ALS/IGF-I Study Group. 1996. Recombinant human insulin-like growth factor-I in ALS: description of a double-blind, placebo-controlled study. Neurology 47 (suppl. 2):S93-S95.
Mitsumoto, H., Ikeda, K., Klinkosz, B., Cedarbaum, J. M., Wong, V., and Lindsay, R. M. 1994. CNTF and BDNF cotreatment arrests in loss of motor function in Wobbler mice. Science 265:1107–1110.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mutoh, T., Sobue, G., Hamano, T. et al. Decreased Phosphorylation Levels of TrkB Neurotrophin Receptor in the Spinal Cords from Patients with Amyotrophic Lateral Sclerosis. Neurochem Res 25, 239–245 (2000). https://doi.org/10.1023/A:1007575504321
Issue Date:
DOI: https://doi.org/10.1023/A:1007575504321