Abstract
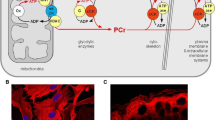
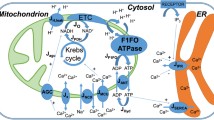
Transmission of energetic signals to membrane sensors, such as the ATP-sensitive K+ (KATP) channel, is vital for cellular adaptation to stress. Yet, cell compartmentation implies diffusional hindrances that hamper direct reception of cytosolic energetic signals. With high intracellular ATP levels, KATP channels may sense not bulk cytosolic, but rather local submembrane nucleotide concentrations set by membrane ATPases and phosphotransfer enzymes. Here, we analyzed the role of adenylate kinase and creatine kinase phosphotransfer reactions in energetic signal transmission over the strong diffusional barrier in the submembrane compartment, and translation of such signals into a nucleotide response detectable by KATP channels. Facilitated diffusion provided by creatine kinase and adenylate kinase phosphotransfer dissipated nucleotide gradients imposed by membrane ATPases, and shunted diffusional restrictions. Energetic signals, simulated as deviation of bulk ATP from its basal level, were amplified into an augmented nucleotide response in the submembrane space due to failure under stress of creatine kinase to facilitate nucleotide diffusion. Tuning of creatine kinase-dependent amplification of the nucleotide response was provided by adenylate kinase capable of adjusting the ATP/ADP ratio in the submembrane compartment securing adequate KATP channel response in accord with cellular metabolic demand. Thus, complementation between creatine kinase and adenylate kinase systems, here predicted by modeling and further supported experimentally, provides a mechanistic basis for metabolic sensor function governed by alterations in intracellular phosphotransfer fluxes.
Similar content being viewed by others
References
Aliev MK, Saks VA: Compartmentalized energy transfer in cardiomyocytes: Use of mathematical modeling for analysis of in vivo regulation of respiration. Biophys J 73: 428–445, 1997
O'Rourke B, Ramza BM, Marban E: Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 265: 962–966, 1994
Ventura-Clapier R, Kuznetsov A, Veksler V, Boehm E, Anflous K: Functional coupling of creatine kinases in muscles: Species and tissue specificity. Mol Cell Biochem 184: 231–247, 1998
Hardie DG, Carling D, Carlson M: The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67: 821–855, 1998
Janssen E, Dzeja PP, Oerlemans F, Simonetti AW, Heerschap A, de Haan A, Rush PS, Terjung RR, Wieringa B, Terzic A: Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement. EMBO J 19: 6371–6381, 2000
Dzeja PP, Redfield MM, Burnett JC, Terzic A: Failing energetics in failing hearts. Curr Cardiol Rep 2: 212–217, 2000
Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J: Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science 270: 1166–1170, 1995
Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R: Energetic crosstalk between organelles: Architectural integration of energy production and utilization. Circ Res 89: 153–159, 2001
Saks VA, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, Regitz-Zagrosek V, Fleck E, Seppet E: Intracellular energetic units in red muscle cells. Biochem J 356: 643–657, 2001
Jovanovic A, Jovanovic S, Lorenz E, Terzic A: Recombinant cardiac ATP-sensitive K+ channel subunits confer resistance to chemical hypoxia-reoxygenation injury. Circulation 98: 1548–1555, 1998
Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A: Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci USA 98: 7623–7628, 2001
Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizu T, Seino S, Inagaki N: Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science 292: 1543–1546, 2001
Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S: A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron 16: 1011–1017, 1996
Lorenz E, Terzic A: Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. J Mol Cell Cardiol 31: 425–434, 1999
Clement JP, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J: Association and stoichiometry of KATP channel subunits. Neuron 18: 827–838, 1997
Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM: Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature 387: 179–183, 1997
Drain P, Li L, Wang J: KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc Natl Acad Sci USA 95: 13953–13958, 1998
Shyng SL, Ferrigni T, Nichols CG: Regulation of KATP channel activity by diazoxide and MgADP: Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol 110: 643–654, 1997
Ashcroft FM, Gribble FM: Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci 21: 288–294, 1998
Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, Aguilar-Bryan L, Permutt MA, Bryan J: Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272: 1785–1787, 1996
Matsuo M, Tanabe K, Kioka N, Amachi T, Ueda K: Different binding properties and affinities for ATP and ADP among sulfonylurea receptor subtypes, SUR1, SUR2A, and SUR2B. J Biol Chem 275: 28757–28763, 2000
Bienengraeber M, Alekseev AE, Abraham MR, Carrasco AJ, Moreau C, Vivaudou M, Dzeja PP, Terzic A: ATPase activity of the sulfonylurea receptor: A catalytic function for the K-ATP channel complex. FASEB J 14: 1943–1952, 2000
Zingman LV, Hodgson DM, Bienengraeber M, Karger AB, Kathmann EC, Alekseev AE, Terzic A: Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J Biol Chem 277: 14206–14210, 2002
Zingman LV, Alekseev AE, Bienengraeber M, Hodgson D, Karger AB, Dzeja PP, Terzic A: Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron 31: 233–245, 2001
Lederer WJ, Nichols CG: Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol 419: 193–211, 1989
Weiss JN, Venkatesh N, Lamp ST: ATP-sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol 447: 649–673, 1992
Bittl JA, DeLayre J, Ingwall JS: Rate equation for creatine kinase predicts the in vivo reaction velocity: 31P NMR surface coil studies in brain, heart, and skeletal muscle of the living rat. Biochemistry 26: 6083–6090, 1987
Saks VA, Aliev MK: Is there the creatine kinase equilibrium in working heart cells? Biochem Biophys Res Commun 227: 360–367, 1996
Weiss JN, Lamp ST: Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science 238: 67–69, 1988
Weiss JN, Venkatesh N: Metabolic regulation of cardiac ATP-sensitive K+ channels. Cardiovasc Drugs Ther 7: 499–505, 1993
Lederer WJ, Niggli E, Hadley RW: Sodium-calcium exchange in excitable cells: Fuzzy space. Science 248: 283, 1990
Dzeja PP, Terzic A: Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J 12: 523–529 1998
Elvir-Mairena JR, Jovanovic A, Gomez LA, Alekseev AE, Terzic A: Reversal of the ATP-liganded state of ATP-sensitive K+ channels by adenylate kinase activity. J Biol Chem 271: 31903–31908, 1996
Abraham MR, Selivanov VA, Hodgson DM, Pucar D, Zingman LV, Wieringa B, Dzeja PP, Alekseev AE, Terzic A: Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J Biol Chem 277: 24427–24434, 2002
Dzeja PP, Vitkevicius KT, Redfield MM, Burnett JC, Terzic A: Adenylate kinase-catalyzed phosphotransfer in the myocardium: Increased contribution in heart failure. Circ Res 84: 1137–1143, 1999
Olson LK, Schroeder W, Robertson RP, Goldberg ND, Walseth TF: Suppression of adenylate kinase catalyzed phosphotransfer precedes and is associated with glucose-induced insulin secretion in intact HIT-T15 cells. J Biol Chem 271: 16544–16552, 1996
Sasaki N, Sato T, Marban E, O'Rourke B: ATP consumption by uncoupled mitochondria activates sarcolemmal KATP channels in cardiac myocytes. Am J Physiol 280: H1882–H1888, 2001
Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A: Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J 16: 102–104, 2002
Nichols CG, Lederer WJ: The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol 423: 91–110, 1990
Chazov EI, Smirnov VN, Saks VA, Rosenshtraukh LV, Lipina NV, Levitsky DO: Energy metabolism and ion fluxes across cardiac membranes. Adv Myocardiol 1: 139–153, 1980
Kabakov AY: Activation of KATP channels by Na/K pump in isolated cardiac myocytes and giant membrane patches. Biophys J 75: 2858–2867, 1998
Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW: Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol 116: 147–161, 2000
Pucar D, Dzeja PP, Bast P, Juranic N, Macura S, Terzic A: Cellular energetics in the preconditioned state: Protective role for phosphotransfer reactions captured by 18O-assisted 31P NMR. J Biol Chem 276: 44812–44819, 2001
Lawson JW, Veech RL: Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem 254: 6528–6537, 1979
Jacobus WE: Theoretical support for the heart phosphocreatine energy transport shuttle based on the intracellular diffusion limited mobility of ADP. Biochem Biophys Res Commun 133: 1035–1041, 1985
Dzeja PP, Zeleznikar RJ, Goldberg ND: Suppression of creatine kinase-catalyzed phosphotransfer results in increased phosphoryl transfer by adenylate kinase in intact skeletal muscle. J Biol Chem 271: 12847–12851, 1996
Pucar D, Janssen E, Dzeja PP, Juranic N, Macura S, Wieringa B, Terzic A: Compromised energetics in the adenylate kinase AK1 gene knockout heart under metabolic stress. J Biol Chem 275: 41424–41429, 2000
Dzeja PP, Bortolon R, Perez-Terzic C, Holmuhamedov EL, Terzic A: Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc Natl Acad Sci USA 99: 10156–10161, 2002
Decking UK, Reffelmann T, Schrader J, Kammermeier H: Hypoxia-induced activation of KATP channels limits energy depletion in the guinea pig heart. Am J Physiol 269: H734–H742, 1995
Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A: Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA 99: 13278–13283, 2002
Pucar D, Bast P, Gumina RJ, Lim L, Drahl C, Juranic N, Macura S, Janssen E, Wieringa B, Terzic A, Dzeja PP. Adenylate kinase AK1 knockout heart: Energetics and functional performance under ischemia-reperfusion. Am J Physiol 283: H776–H782, 2002
Ghosh MC, Jencks WP: Phosphorylation of the sodium-potassium adenosinetriphosphatase with adenosine triphosphate and sodium ion that requires subconformations in addition to principal E1 and E2 conformations of the enzyme. Biochemistry 35: 12587–12590, 1996
Joubert F, Mazet JL, Mateo P, Hoerter JA: 31P NMR detection of subcellular creatine kinase fluxes in the perfused rat heart: Contractility modifies energy transfer pathways. J Biol Chem 277: 18469–18476, 2002
Gogelein H, Hartung J, Englert HC: Molecular basis, pharmacology and physiological role of cardiac KATP channels. Cell Physiol Biochem 9: 227–241, 1999
Kusuoka H, Inoue M, Tsuneoka Y, Watari H, Hori M, Abe H: Augmented energy consumption during early systole as a mechanism of cyclical changes in high-energy phosphates in myocardium assessed by phosphorus nuclear magnetic resonance. Jpn Circ J 49: 1099–107, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Selivanov, V.A., Alekseev, A.E., Hodgson, D.M. et al. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: Amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem 256, 243–256 (2004). https://doi.org/10.1023/B:MCBI.0000009872.35940.7d
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000009872.35940.7d