Abstract
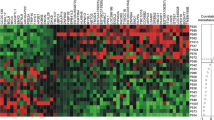
TGFβ/Smad signaling pathway members are potent tumor suppressors for many types of cancers. We hypothesize that breast tumors differentially express these genes and that this expression pattern plays a role in the proliferation of breast cancer. We examined the mRNA levels of TIEG, Smad7, Smad2, and Bard1 using real-time RT/PCR in 14 normal breast, five non-invasive, 57 invasive (including 29 with outcome data), and five metastatic breast tumor tissues. TIEG and Smad7 mRNA levels were lower in non-invasive tumors compared to normal breast tissues. TIEG, Bard1, and Smad2 mRNA levels were lower in invasive cancers compared to normal breast tissues. In addition, TIEG, Smad2, and Bard1, provided discriminatory ability to potentially distinguish between normal and tumor samples, N− and N+ tumors, and N-/good (no recurrence for at least 5 years) and N-/bad (recurrence within 3 years) outcome patients. TIEG mRNA levels accurately discriminated between normal breast tissue and primary tumors with a sensitivity and specificity of 96 and 93%, respectively. TIEG, in combination with Smad2, distinguished between N+ and N− primary tumors with a sensitivity and specificity of 75 and 85%, respectively. TIEG in combination with Bard1 discriminated between N-/bad outcome from N-/good tumors with a sensitivity and specificity of 83 and 82%, respectively. Our results support the hypothesis that the differential gene expression of TIEG, Smad2, and Bard1, which are tumor suppressor genes, plays a significant role in the proliferation of breast cancer. Further investigation is necessary to validate the ability of these genes to discriminate between different populations of breast cancer patients.
Similar content being viewed by others
References
Valverius EM, Walker-Jones D, Bates SE, Stampfer MR, Clark R, McCormick F, Dickson RB, Lippman ME: Production of and responsiveness to transforming growth factor-beta in normal and oncogene-transformed human mammary epithelial cells. Cancer Res 49: 6269–6274, 1989
Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, Dickson RB: Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell 48: 417–428, 1987
Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE: DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 271: 350–353, 1996
Kim SK, Fan Y, Papadimitrakopoulou V, Clayman G, Hittelman WN, Hong WK, Lotan R, Mao L: DPC4, a candidate tumor suppressor gene, is altered infrequently in head and neck squamous cell carcinoma. Cancer Res 56: 2519–2521, 1996
Schutte M, Hruban RH, Hedrick L, Cho KR, Nadasdy GM, Weinstein CL, Bova GS, Isaacs WB, Cairns P, Nawroz H, Sidransky D, Casero RA, Meltzer PS, Hahn SA, Kern SE: DPC4 gene in various tumor types. Cancer Res 56: 2527–2530, 1996
Takagi Y, Kohmura H, Futamura M, Kida H, Tanemura H, Shimokawa K, Saji S: Somatic alterations of the DPC4 gene in human colorectal cancers in vivo. Gastroenterology 111: 1369–1372, 1996
Kleeff J, Ishiwata T, Maruyama H, Friess H, Truong P, Buchler MW, Falb D, Korc M: The TGF-beta signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene 18: 5363–5372, 1999
Kleeff J, Maruyama H, Friess H, Buchler MW, Falb D, Korc M: Smad6 suppresses TGF-beta-induced growth inhibition in COLO-357 pancreatic cancer cells and is overexpressed in pancreatic cancer. Biochem Biophys Res Commun 255: 268–273, 1999
Luo K, Stroschein SL, Wang W, Chen D, Martens E, Zhou S, Zhou Q: The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling. Genes Dev 13: 2196–2206, 1999
Stroschein SL, Wang W, Zhou S, Zhou Q, Luo K: Negative feedback regulation of TGF-beta signaling by the SnoN oncoprotein. Science 286: 771–774, 1999
Colmenares C, Stavnezer E: The ski oncogene induces muscle differentiation in quail embryo cells. Cell 59: 293–303, 1989
Boyer PL, Colmenares C, Stavnezer E, Hughes SH: Sequence and biological activity of chicken snoN cDNA clones. Oncogene 8: 457–466, 1993
Subramaniam M, Harris SA, Oursler MJ, Rasmussen K, Riggs BL, Spelsberg TC: Identification of a novel TGF? regulated gene encoding a putative zinc finger protein in human osteoblasts. Nucl Acids Res 23: 4907–4912, 1995
Tachibana I, Imoto M, Adjei PN, Gores GJ, Subramaniam M, Spelsberg TC, Urrutia R: Overexpression of the TGF?-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J Clin Invest 99: 2365–2374, 1997
Subramaniam M, Hefferan TE, Tau KR, Peus D, Pittelkow M, Jalal S, Riggs BL, Roche P, Spelsberg TC: Tissue, cell type, and breast cancer stage-specific expression of a TGF? inducible early transcription factor gene. J Cell Biochem 68: 226–236, 1998
Fautsch MP, Vrabel A, Subramaniam M, Hefferen TE, Spelsberg TC, Wieben ED: TGF-? inducible early gene (TIEG) also codes for early growth response alpha (EGR?): Evidence of multiple transcripts from alternate promoters. Genomics 51: 408–416, 1998
Fautsch MP, Vrabel A, Rickard D, Subramaniam M, Spelsberg TC, Wieben ED: Characterization of the mouse TGF-? inducible early gene (TIEG): conservation of exon and transcriptional regulatory sequences with evidence of additional transcripts. Mamm Genome 9: 838–842, 1998
Cook T, Gebelein B, Belal M, Mesa K, Urrutia R: Three conserved transcriptional repressor domains are a defining feature of the TIEG subfamily of Sp1-like zinc finger proteins. J Biol Chem 274: 29500–29504, 1999
Hefferan TE, Reinholz GG, Rickard DJ, Johnsen SA, Waters KM, Subramaniam M, Spelsberg TC: Overexpression of a nuclear protein, TIEG, mimics transforming growth factor-beta action in human osteoblast cells. J Biol Chem 275: 20255–20259, 2000
Ribeiro A, Bronk SF, Roberts PJ, Urrutia R, Gores GJ: The transforming growth factor beta(1)-inducible transcription factor TIEG1, mediates apoptosis through oxidative stress. Hepatology 30: 1490–1497, 1999
Chalaux E, Lopez-Rovira T, Rosa JL, Pons G, Boxer LM, Bartrons R, Ventura F: A zinc-finger transcription factor induced by TGF-beta promotes apoptotic cell death in epithelial Mv1Lu cells. FEBS Lett 457: 478–482, 1999
Johnsen SA, Subramaniam M, Janknecht R, Spelsberg TC: TGF? inducible early gene enhances TGF?/Smad dependent transcriptional responses. Oncogene 21: 5783–5790, 2002
Johnsen SA, Subramaniam M, Monroe DG, Janknecht R, Spelsberg TC: Modulation of TGF?/Smad transcriptional responses through targeted degradation of the TGF? inducible early gene-1 by the human seven in absentia homologue. J Biol Chem 277: 30754–30759, 2002
Johnsen SA, Subramaniam M, Katagiri T, Janknecht R, Spelsberg TC: Transcriptional regulation of Smad2 is required for enhancement of TGF?/Smad signaling by TGF? inducible early gene. J Cell Biochem 87: 233–241, 2002
Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P: Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signaling. Nature 389: 631–635, 1997
Baer R, Ludwig T: The BRCA1/Bard1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr Opin Gen Devel 12: 86–91, 2002
Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T: The RING heterodimer BRCA1-Bard1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem 276: 14537–14540, 2001
Irminger-Finger I, Leung W-C, Li J, Dubois-Dauphin M, Harb J, Feki A, Jefford CE, Soriano JV, Jaconi M, Montesano R, Krause K-H: Identification of BARD1 as mediator between proapoptotic stress and p53-dependent apoptosis. Mol Cell 8: 1255–1266, 2001
Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ, Deshler A, Fulton S, Hendricks CB, Kemeny M, Kornblith AB, Louis TA, Markman M, Mayer R, Roter D: National Institutes of Health Consensus Development Conference Statement: Adjuvant Therapy for Breast Cancer, 1-3 November 2000. J Natl Cancer Inst 93: 979–989, 2001
Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer, St. Gallen, Switzerland, February 2001.
West M, Blanchette C, Dressman H, Huang E, Ishida S, Spang R, Zuzan H, Olson JA, Marks JR, Nevins JR: Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci USA 98: 11462–11467, 2001
Van de Vijver MJ, Yudong D, van't Veeer LJ, Dai H, Hart AAM, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R: A gene-expression signature as a predictor of survival in breast cancer. New Engl J Med 347: 999–2009, 2002
Holland PM, Abramson RD, Watson R, Gelfand DH: Detection of specific polymerase chain reaction products by utilizing the 5´ to 3´ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA 88: 7276–7280, 1991
Heid CA, Stevens J, Livak KJ, Williams PM: Real time quantitative PCR. Genome Res 6: 986–994, 1996
Reimer T, Koczan D, Müller H, Friese K, Krause A, Thiesen H, Gerber B: Human chorionic gonadotropin-? transcripts correlate with progesterone receptor values in breast carcinomas. J Mol Endocrinol 24: 33–41, 2000
Bièche I, Laurendau I, Tozlu S, Olivi M, Vidaud D, Lidereau R, Vidaud M: Quantitation of MYC gene expression in sporadic breast tumors with a real-time reverse transcription-PCR assay. Cancer Res 59: 2759–2765, 1999
Bièche I, Onody P, Laurendeau I, Olivi M, Vidaud D, Lidereau R, Vidaud M: Real-time reverse transcription-PCR assay for future management of ERBB2-based clinical applications. Clin Chem 45: 1148–1156, 1999
Reinholz MM, Iturria SJ, Ingle JN, Roche PC: Differential gene expression of TGF-? family members and osteopontin in breast tumor tissue: analysis by real-time quantitative PCR. Breast Cancer Res Tr 74: 255–269, 2002
Nakao A, Roijer E, Imamura T, Souchelnytskyi S, Stenman G, Heldin C-H, Dijke PT: Identification of Smad2, a human Mad-related protein in the transforming growth factor ? signaling pathway. J Biol Chem 272: 2896–2900, 1997
Topper JN, Cai J, Qui Y, Anderson KR, Xu Y-Y, Deeds JD, Feeley R, Gimeno CJ, Woolf EA, Tayber O, Mays GG, Sampson BA, Schoen FJ, Gimbrone MA, Falb D: Vascular MADS: Two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc Natl Acad Sci USA 94: 9314–9319, 1997
Wu LC, Wang ZW, Tsan JT, Spillman MA, Phung A, Xu XL, Yang M-CW, Hwang L-Y, Bowcock AM, Baer R: Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet 14: 430–440, 1996
Dirnhofer S, Berger C, Untergasser G, Geley S, Berger P: Human ?-actin retropseudogenes interfere with RT-PCR. TIG 11: 380–381, 1995
Xie W, Mertens JC, Reiss DJ, Rimm DL, Camp RL, Haffty BG, Reiss M: Alterations of Smad signaling in human breast carcinoma are associated with poor outcome. A tissue microarray study. Cancer Res 62: 497–505, 2002
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reinholz, M.M., An, MW., Johnsen, S.A. et al. Differential Gene Expression of TGFβ Inducible Early Gene (TIEG), Smad7, Smad2 and Bard1 in Normal and Malignant Breast Tissue. Breast Cancer Res Treat 86, 75–88 (2004). https://doi.org/10.1023/B:BREA.0000032926.74216.7d
Issue Date:
DOI: https://doi.org/10.1023/B:BREA.0000032926.74216.7d