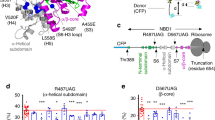
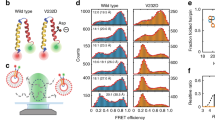
Abstract
Many human diseases arise as a result of mutations within genes encoding essential proteins. In many cases, the mutations are not so severe as to render the protein biologically inactive. Rather, the mutations oftentimes result in only subtle protein-folding abnormalities. In the case of the CFTR protein, a mutation leading to the loss of a single amino acid is responsible for the diseased state in the majority of individuals with cystic fibrosis. Here the newly synthesized mutant CFTR protein, missing a phenylalanine residue at position 508 (ΔF508 CFTR), is unable to transit from the endoplasmic reticulum to the plasma membrane, where it functions as a regulator of chloride transport. All of the available evidence indicate that the newly synthesized ΔF508 CFTR protein adopts a slightly altered conformation and therefore is retained at the level of the endoplasmic reticulum, ostensibly by the actions of the cellular quality control system. Because the mutant protein is capable of functioning as a chloride channel, developing ways to elicit its release out of the ER and to the plasma membrane has important clinical implications. Herein, we discuss our recent studies showing that the protein folding defect associated with the ΔF508 CFTR mutation, as well as a number of other temperature-sensitive mutations, can be overcome by strategies designed to influence protein folding inside the cell. Specifically we show that a number of low-molecular-weight compounds, all of which are known to stabilize proteins in their native conformation, are effective in rescuing the folding and/or processing defects associated with different mutations that oftentimes lead to human disease.
Similar content being viewed by others
REFERENCES
Alton, E. W., Middleton, P. G., Caplan, N. J., Smith, S. N., Steel, D. M., Munkonge, F. M., Jeffery, P. K., Geddes, D. M., Hart, S. L., Williamson, R., Fasold, K. I., Miller, A. DI, Dickinson, P., Stevenson, B. J., McLachlan, G., Dorin, J. R., and Porteous, D. J. (1993). Nature Genet. 5, 135–142.
Amara J. F., Cheng, S. H., and Smith, A. E. (1992). Trends Cell Biol. 2, 145–149.
Back, J. F., Oakenfull, D., and Smith, M. B. (1979). Biochemistry 18, 5191–5199.
Bear, C. E., Jensen, T. J., and Riordan, J. R. (1992). Biophys. J. A127.
Becq, F., Jensen, T. J., Chang, X. B., Savoia, A., Rommens, J. M., Tsui, L. C., Buchwald, M., Riordan, J. R., and Hanrahan, J. W. (1994). Proc. Natl. Acad. Sci. 91, 9160–9164.
Becq, F., B. Verrier, X. B. Chang, J. R. Riordan and J. W. Hanrahan. (1996). J. Biol. Chem. 271, 16171–16179.
Brown, C. R., Hong-Brown, L. Q., Biwersi, J., Verkman, A. S., and Welch, W. J. (1996). Cell Stress Chap. 1, 117–125.
Brown, C. R., Hong-Brown, L. Q., and Welch, W. J. (1997). J. Clin. Invest., 99, 1432–1444.
Burg, M. B. (1995). Am. J. Physiol. 268, F983–F996.
Bychkova V. E., and Ptitsyn, O. B. (1995). FEBS Let. 359, 6–8.
Caplan, N. J., Alton, E. W., Middleton, P. G., Dorin, J. R., Stevenson, B. J., Gao, X., Durham, S. R., Jeffery, P. K., Hodson, M. E., Coutelle, C., Huang, L., Porteous, D. J., Williamson, R., and Geddes, D. M. (1995). Nature Med. 1, 39–46.
Cheng, S. H., Gregory, R. J., Marshall, J., Paul, S., Souza, D. W., White, G. A., O'Riordan, C. R., and Smith, A. E. (1990). Cell 63, 827–834.
Cheng, S. H., Fang, S. L., Zabner, J., Marshall, J., Piraino, S., Schiavi, S. C., Jefferson, D. M., Welsh, M. J., and Smith, A. E. (1995). Am. J. Physiol. 268, L615–L624.
Chowdary, D. R., Dermody, J. J., Jha, K. K., and Ozer, H. L. (1994). Mol. Cell. Biol. 14, 1997–2003.
Denning, G. M., Anderson, M. P., Amara, J. F., Marshallo, J., Smith, A. E., and Welsh, M. J. (1992). Nature 358, 761–764.
Drumm, M. L., Wilkinson, D. S., Smit, L. S., Worrell, R. T., and Strong, T. V., Frizzell, R. A., Dawson, D. C., and Collins, F. S. (1991). Science 254, 1797–1799.
Edington, B. V., Whelan, S. A., and Hightower, L. E. (1989). J. Cell. Physiol. 139, 219–228.
Eidelman, O., Guay-Broder, C., van Galen, P. J. M., Jacobson, K., Fox, C., Turner, R. J., Cabantchik, Z. I., and Pollard, H. B. (1992). Proc. Natl. Acad. Sci. USA 89, 5562–5566.
Garcia-Perez, A., and Burg, M. B. (1991). Physiol. Rev. 71, 1081–1115.
Germsla, S. Y., and Stuur, E. R. (1972). Int. J. Pept. Protein Res. 4, 372–378.
Gekko, K., and Koga, S. (1983). J. Biochem. 94, 199–208.
Gekko, K., and Timasheff, S. N. (1981a). Biochemistry 20, 4667–4676.
Gekko, K. and Timasheff, S. N. (1981b). Biochemistry 20, 4677–4686.
Guay-Broder, C., Jacobson, K. A., Barnoy, S. Cabantchik, Z. I., Guggino, W. B., Zeitlin, P. L., Turner, R. J., Vergara, L., Eidelman, O., and Pollard, H. B. (1995). Biochemistry 34, 9079–9087.
Hamosh, A., Trapnelllll, B. C., Zeitlin, P. L., Montrose-Rafizadeh, C., Rosenstein, B. I., Crystal, R. G., and Cutting, G. R. (1991). J. Clin. Invest. 88, 1880–1885.
Henle, K. J., Peck, J. W., and Higashikubo, R. (1983). Cancer Res. 43, 1624–1633.
Hawthorne, D. C., and Friss, J. (1964). Genetics 50, 829–839.
Howard, M., Frizzell, R. A., and Bedwell, D. M. (1996). Nature Med. 2, 467–469.
Illek, B., Fisher, H., and Machen, T. E. (1996). Am J. Physiol. 270, C265–275.
Jacobson, K. A., Guay-Broder, C. van Galen, P. J. M., Rodrigues, C., Melman, N., Jacobson, M. A., Eidelman, O., and Pollard, H. (1995). Biochemistry 34, 9088–9094.
Kelley, T. J., Al-Nakkash, L., Cotton, C. U., and Drumm, M. L. (1996). J. Clin. Invest. 98, 513–520.
Kerem, B. S., Rommens, J. M., Buchanan, J. A., Markiewicz, D., Cox, T. K., Chakravarti, A., Buchwald, M., and Tsui, L. C. (1989). Science 245, 1073–1080.
Kottgen, M., Busch, A. E., Hug, M. J., Greger, R., and Kunzelmann, K. (1996). Pflug. Arch. Eur. J. Physiol. 431, 549–555.
Lee, E. R., Marshall, J., Siegel, C. S., Jiang, C., Yew, N. S., Nichols, M. R., Nietupski, J. B., Ziegler, R. J., Lane M. B., Wang, K. X., Wan, N. C., Scheule, R. K., Harris, D. J., Smith, A. E., and Cheng, S. H. (1996). Hum. Gene Ther. 7, 1701–1717.
Li, C., Ramjeesingh, M., Reyea, E., Jensen, T., Chang, X., Rommens, J. M., and Bear, C. E. (1993). Nature Genet. 3, 311–316.
Lin, P. S., Kwock, L., and Hefter, K. (1981). J. Cell. Physiol. 108, 439–448.
Martinez, J., Georgoff, I., Martinez, J., and Levine, A. J. (1991). Genes Dev. 5, 151–159.
Pind, S., Riordan, J. R., and Williams, D. B. (1994). J. Biol. Chem. 269, 12784–12788.
Riordan, J. R., Rommens, J. M., Keren, B. S., Alon, N., Rozmahel, R., Grzelczak, Z., Zielenski, J., Lok, S., Plavsic, N., Chou, J. L., Drumm, M. L., Iannuzzi, M. C., Collins, F. S., and Tsui, L. C. (1989). Science 245, 1066–1073.
Rommens, J. M., Iannuzzi, M. C., Kerem, B. S., Drumm, M. L., Melmer, G., Dean, M., Rozmahel, R., Cole, J. L., Kennedy, D., Hidaka, N., Zsiza, M., Buchwald, M., Riordan, J. R., Tsui, L. C., and Collins, F. S. (1989). Science 245, 1059–1065.
Sato, S., Ward, C. L., Krouse, M. E., Wine, J. J., and Kopito, R. R. (1996). J. Biol. Chem. 271, 635–638.
Schein, C. H. (1990). Bio/Technol. 8, 308–316.
Somero, G. (1986). Am. J. Physiol. 251, R197–R213.
Thomas, P. J., Qu, B. H., and Pedersen, P. L. (1995). Trends Biochem. Sci. 20, 456–459.
Tatzelt, J., Pruisner, S., and Welch, W. J. (1996). EMBO J. 15, 6363–6373.
Welch, W. J., and Brown, C. R. (1996). Cell Stress Chap. 1, 109–115.
Welsh, M. J., and Smith, A. E. (1993). Cell 13, 1251–1254.
Yancey, P. H., Clark, M. E., Hand, S. C., Bowlus, R. D., and Somero, G. N. (1982). Science 217, 1214–1222.
Yang, Y., Janich, S., Cohn, J. A., and Wilson, J. M. (1993). Proc. Natl. Acad. Sci. USA 90, 9480–9484.
Zabner, J. B., Ramsey, W., Meeker, D. P., Aitken, M. L., Balfour, R. P., Gibson, R. L., Launspach, J., Moscicki, R. A., Richards, S. M., Standaert, T. A., Williams-Warren, J., Wadsworth, S. C., Smith, A. E., and Welsh, M. J. (1996). J. Clin. Invest. 97, 1504–1511.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, C.R., Hong-Brown, L.Q. & Welch, W.J. Strategies for Correcting the AF508 CFTR Protein-Folding Defect. J Bioenerg Biomembr 29, 491–502 (1997). https://doi.org/10.1023/A:1022491124939
Issue Date:
DOI: https://doi.org/10.1023/A:1022491124939