Abstract
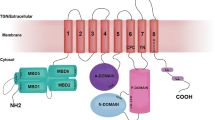
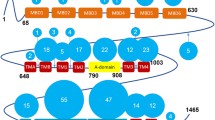
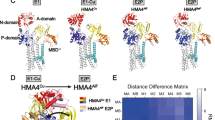
Wilson's disease protein (WNDP) is a product of a gene ATP7B that is mutated in patients with Wilson's disease, a severe genetic disorder with hepatic and neurological manifestations caused by accumulation of copper in the liver and brain. In a cell, WNDP transports copper across various cell membranes using energy of ATP-hydrolysis. Copper regulates WNDP at several levels, modulating its catalytic activity, posttranslational modification, and intracellular localization. This review summarizes recent studies on enzymatic function and copper-dependent regulation of WNDP. Specifically, we describe the molecular architecture and major biochemical properties of WNDP, discuss advantages of the recently developed functional expression of WNDP in insect cells, and summarize the results of the ligand-binding studies and molecular modeling experiments for the ATP-binding domain of WNDP. In addition, we speculate on how copper binding may regulate the activity and intracellular distribution of WNDP, and what role the human copper chaperone Atox1 may play in these processes.
Similar content being viewed by others
REFERENCES
Arnesano, F., Banci, L., Bertini, I., Ciofi-Baffoni, S., Molteni, E., Huffman, D. L., and O'Halloran, T. V. (2002). Genome Res. 12(2), 255–271.
Bissig, K. D., Voegelin, T. C., and Solioz, M. (2001). FEBS Lett. 507(3), 367–370.
Bissig, K. D., Wunderli-Ye, H., Duda, P. W., and Solioz, M. (2001). Biochem. J. 357, 217–223.
Capieaux, E., Rapin, C., Thines, D., Dupont, Y., and Goffeau, A. (1993). J. Biol. Chem. 268(29), 21895–21900.
Carmichael, P. L., Hewer, A., Osborne, M. R., Strain, A. J., and Phillips, D. H. (1995). Mutat. Res. 326(2), 235–243.
DiDonato, M., Hsu, H. F., Narindrasorasak, S., Que, L., Jr., and Sarkar, B. (2000). Biochemistry 39(7), 1890–1896.
DiDonato, M., Narindrasorasak, S., Forbes, J. R., Cox, D.W., and Sarkar, B. (1997). J. Biol. Chem. 272(52), 33279–33282.
Efremov R. G., Kosinsky Yu. A., Lutsenko S. V. (manuscript in preparation).
Forbes, J. R., Hsi, G., and Cox, D. W. (1999). J. Biol. Chem. 274(18), 12408–12413.
Gatto, C., Wang, A. X., and Kaplan, J. H. (1998). J. Biol. Chem. 273(17), 10578–10585.
Gitschier, J., Moffat, B., Reilly, D., Wood, W. I., and Fairbrother, W. J. (1998). Nat. Struct. Biol. 5(1), 47–54.
Gu, M., Cooper, J. M., Butler, P., Walker, A. P., Mistry, P. K., Dooley, J. S., and Schapira, A. H. (2000). Lancet 356(9228), 469–474.
Hamza, I., Faisst, A., Prohaska, J., Chen, J., Gruss, P., and Gitlin, J. D. (2001). Proc. Natl. Acad. Sci. USA 98(12), 6848–6852.
Harrison, M. D., Jones, C. E., and Dameron, C. T. (1999). J. Biol. Inorg. Chem. 4, 145–153.
Iida, M., Terada, K., Sambongi, Y., Wakabayashi, T., Miura, N., Koyama, K., Futai, M., and Sugiyama, T. (1998). FEBS Lett. 428(3), 281–285.
Kuntz, I. D. (1992). Science 257(5073), 1078–1082.
Linder, M. C., and Hazegh-Azam, M. (1996). Am. J. Clin. Nutr. 63(5), 797S–811S.
Luthy R., Bowie J. U., Eisenberg D. (1992). Assessment of protein models with three-dimensional profiles. Nature 356, 83–85.
Lutsenko, S., Petrukhin, K., Cooper, M. J., Gilliam, C. T., and Kaplan, J. H. (1997). J. Biol. Chem. 272(30), 18939–18944.
Majumdar, R., Al Jumah, M., Al Rajeh, S., Fraser, M., Al Zaben, A., Awada, A., Al Traif, I., and Paterson, M. (2000). J. Neurol. Sci. 179 (Suppl. 1/2), 140–143.
Moutin, M. J., Cuillel, M., Rapin, C., Miras, R., Anger, M., Lompre, A. M., and Dupont, Y. (1994). Biol. Chem. 269(15), 11147–11154.
Nair, J., Carmichael, P. L., Fernando, R. C., Phillips, D. H., Strain, A. J., and Bartsch, H. (1998). Cancer Epidemiol. Biomarkers Prev. 7(5), 435–440.
O'Halloran, T. V., and Culotta, V. C. (2000). J. Biol. Chem. 275(33), 25057–25060.
Payne, A. S., Kelly, E. J., and Gitlin, J. D. (1998). Proc. Natl. Acad. Sci. USA 95(18), 10854–10859.
Petris, M. J., Camakaris, J., Greenough, M., LaFontaine, S., and Mercer, J. F. (1998). Hum. Mol. Genet. 7(13), 2063–2071.
Petrukhin, K., Lutsenko, S., Chernov, I., Ross, B. M., Kaplan, J. H., and Gilliam, T. C. (1994). Hum. Mol. Genet. 3(9), 1647–1656.
Rae, T. D., Schmidt, P. J., Pufahl, R. A., Culotta, V. C., and O'Halloran, T. V. (1999). Science 284(5415), 805–808.
Roelofsen, H., Wolters, H., Van Luyn, M. J., Miura, N., Kuipers, F., and Vonk, R. J. (2000). Gastroenterology 119(3), 782–793.
Sali, A., and Overington, J. P. (1994). Protein Sci. 3(9), 1582–1596.
Schaefer, M., Hopkins, R. G., Failla, M. L., and Gitlin, J. D. (1999). Am. J. Physiol. 276, G639–G646.
Scheinberg, I. H., and Sternlieb, I. (1984). In Major Problems in Internal Medicine, Vol. 23 (Smith, L. H., Jr., ed.), W. B. Saunders, Philadelphia Suzuki, M., and Gitlin, J. D. (1999). Pediatr. Int. 41(4), 436-442.
Sweadner, K. J., and Donnet, C. (2001). Biochem. J. 356(Pt 3), 685–704.
Tanzi, R. E., Petrukhin, K., Chernov, I., Pellequer, J. L., Wasco, W., Ross, B., Romano, D. M., Parano, E., Pavone, L., Brzustowicz, L.M., Devoto M., Peppercorn, J., Bush, A. I., Sternlieb, I., Pirastu, M., Gusella, J. F., Evgrafov, O., Penchaszadeh, G. K., Honig, B., Edelman, I. S., Soares, M. B., Scheinberg, I. H., and Gilliam, T. C. (1993). Nat. Genet. 5(4), 344–350.
Terada, K., Nakako, T., Yang, X. L., Iida, M., Aiba, N., Minamiya, Y., Nakai, M., Sakaki, T., Miura, N., and Sugiyama, T. (1998). J. Biol. Chem. 273(3), 1815–1820.
Thomas, G. R., Forbes, J. R., Roberts, E. A., Walshe, J. M., and Cox, D. W. (1995). Nat. Genet. 9(2), 210–217. 362 Lutsenko, Efremov, Tsivkovskii, andWalker
Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000). Nature 405, 647–655
Tsivkovskii, R., Eisses, J. F., Kaplan, J. H., and Lutsenko, S. (2002). J. Biol. Chem. 277(2), 976–983.
Tsivkovskii, R., MacArthur, B. C., and Lutsenko, S. (2001). J. Biol. Chem. 276(3), 2234–2242.
Vanderwerf, S. M. A. L. S. (2002). In Biochemical Society Transactions for Biometals 2002: 3rd International Biometals Symposium, King's College London, UK.
Vanderwerf, S. M., Cooper, M. J., Stetsenko, I. V., and Lutsenko, S. (2001). J. Biol. Chem. 276(39), 36289–36294.
Voskoboinik, I., Greenough, M., La Fontaine, S., Mercer, J. F. B., and Camakaris, J. (2001). Bichem. Biophys. Res. Commun. 9, 966–970.
Walker, J. M., Tsivkovskii, R., and Lutsenko, S. (2002). J. Biol. Chem. 23, 23.
Wernimont, A. K., Huffman, D. L., Lamb, A. L., O'Halloran, T. V., and Rosenzweig, A. C. (2000). Nat. Struct. Biol. 7(9), 766–771.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lutsenko, S., Efremov, R.G., Tsivkovskii, R. et al. Human Copper-Transporting ATPase ATP7B (The Wilson's Disease Protein): Biochemical Properties and Regulation. J Bioenerg Biomembr 34, 351–362 (2002). https://doi.org/10.1023/A:1021297919034
Issue Date:
DOI: https://doi.org/10.1023/A:1021297919034