Abstract
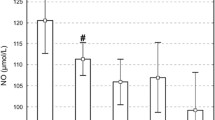
This experimental study was performed to investigate the role of ischemia–reperfusion injury on retinal nitric oxide activity and to determine whether octreotide, the synthetic analogue of natural somatostatin, modifies the nitric oxide activity during retinal ischemia–reperfusion in a quinea pig model. Three groups of seven pigmented male quinea pigs were formed; Control, Ischemia and the Ischemia/Octreotide groups. 90 minutes of pressure-induced retinal ischemia and 24 h of reperfusion were established in the ischemia and ischemia/octreotide groups. Saline for the ischemia group and 50 μg/kg of octreotide for the ischemia/octreotide group were administered intraperitoneally five times with 6-h intervals. At the end of the reperfusion period both eyes of the animals of the three groups were enucleated. One eye of each animal was randomly selected for biochemical assay and the other for histopathological analysis. Retinal nitrate levels were measured and histopathological changes were evaluated in the groups. The mean retinal nitrate levels of the control, ischemia and ischemia/octreotide groups were 157.6±25.2, 106.4±20.1 and 96.4±17.7 μmol/l, respectively. Nitrate levels decreased significantly both in the ischemia (p<0.01) and ischemia/octreotide (p<0.01) groups versus control. In the ischemia group, retinal histopathological changes, which were different from the control group, were prominent edema, polymorphonucleated leukocytes infiltration and vacuolated spaces in the inner retina. No significant change was observed in the histopathological specimens of the ischemia/octreotide group. Significant increase in the thickness of the inner plexiform layer of the retina of the ischemia group was observed versus the control and ischemia/octreotide groups (p<0.01 and p<0.01, respectively).The thickness of the inner plexiform layer of the retina of the ischemia/octreotide group did not change versus the control group. It was concluded that nitric oxide activity decreased during retinal ischemia–reperfusion and, although octreotide prevented the histopathological damage, it could not ameliorate the nitric oxide activity of the retina.
Similar content being viewed by others
References
Choi DW. Glutamate neurotoxicity: A three stage process. In Guidotti A, ed. Neurotoxicity of amino acids. FIDIA Research Foundation Symposium Series. New York: Raven Press, 1991: 235–42
Goldstein IM, Ostwald P, Roth S. Nitric oxide: A review of its role in retinal function and disease. Vision Res 1996; 36: 2979–94.
Hangai M, Yoshimura N, Hiroi K, Mandai M, Honda Y. Inducible nitric oxide synthase in retinal ischemia-reperfusion injury. Exp Eye Res 1996; 63: 501–9.
Yamamato R, Bredt DS, Snyder SH, Stone RA. The localization of nitric oxide synthase in rat eye and related cranial ganglia. Neuroscience 1993; 54: 19–200.
Koch K, Lambrecht H, Haberecht M, Redburn D, Schmith HHHW. Functional coupling of a calcium/calmodulin-dependent nitric oxide synthase an a soluble quanyl cyclase in vertebrate photoreceptor cells. EMBO J 1994; 13: 3312–20.
Perez MTR, Larsson B, Alm P, Anderson KE, Ehinger B. Localization of neuronal nitric oxide synthase-immunoreactivity in rat and rabbit retinas. Brain Res 1995; 104: 207–17.
Chakravarty U, Stitt AW, McNally J, Bailie J, Hoey EM, Duprex P. Nitric oxide synthase activity and expression in retinal capillary endothelial cells and pericytes. Curr Eye Res 1994; 14: 285–94.
Goureau O, Hicks D, Courtois Y, de Kozak Y. Induction and regulation of nitric oxide synthase in retinal Müller glial cells. J Neurochem 1994; 63: 310–7.
Meyer P, Champion C, Schlotzer-Schrehardt U, Flammer J, Haefliger IO. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res 1999; 18: 375–80.
Harrdy P, Dumont I, Bhattacharya M, Hou X, Lachapelle P, Varma D, Chemtob S. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovasc Res 2000; 47: 489–509.
Radomski MW, Palmer RMJ, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA 1990 87; 5193–7.
Engelman DT, Watanabe M, Engelman RM, Rousou JA, Flack JE, Deaton D, Das DK. Constitutive nitric oxide release is impaired after ischemia and reperfusion. J Thorac Cardiovasc Surg 1995; 110: 1047–53.
Araki M, Tanaka M, Hasegawa K, Yokota R, Maeda T, Ishikawa M, Yabuuchi Y, Sasayama S. Nitric oxide inhibition improved myocardial metabolism independent of tissue perfusion during ischemia but not during reperfusion. J Mol Cell Cardiol 2000; 32: 375–84.
Mashiach Elisheva, Shifra Sela, Winaver J, Shasha SM, Kristal B. Renal ischemia- reperfusion injury: contribution of nitric oxide and renal blood flow. Nephron 1998; 80: 45–7.
Kusterer K, Blöchle C, Konrad T, Palitzsch KD, Usadel KH. Rat liver injury induced by hypoxic ischemia and reperfusion: protective action by somatostatin and two derivatives. Regul Pept 1993; 44: 251–6.
Niedermühlbicher M, Wiedermann CJ. Supression of superoxide release from human monocytes by somatostatin related peptides. Regul Pept 1992; 41: 39–47.
Le Q, Zhang X, Zhang J. Effects of sandostatin on gastric mucosal perfusion in rats with portal hypertensive gastropathy. Chung Hua Kan Tsang Ping Tsa Chih 2000; 8: 21–3.
Arias-Diaz J, Vara E, Torres-Molero J, Garcia C, Hernandez J, Balibrea JL. Local production of oxygen free radicals and nitric oxide in rat diaphragm during sepsis: effects of pentoxifylline and somatostatin. Eur J Surg 1997; 163: 619–25.
Chao TC, Cheng HP, Walter RJ. Somatostatin and macrophage function: modulation of hydrogen peroxide, nitric oxide and tumor necrosis factor release. Regul Pept 1995; 58: 1–10.
Borries PN, Borries C. Nitrate determination in biological fluids by an enzymatic onestep assay with nitrate reductase. Clin Chem 1995; 41: 904–7.
Del Maestro RF. An approach to the free radicals in medicine and biology. Acta Physiol Scand 1980; 492: 153–68.
Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxinonenal, malondialdehyde and related aldehydes. Free Radic Biol Med 1991; 11: 81–128.
Comporti M. Lipid peroxidation and biogenic aldehydes: from the identification of 4-hydroxinonenal to further achievements in biopathology. Free Radic Res 1998; 28: 623–35.
Buko VU, Artukevich AA, Ignatenko KV. Aldehydic products of lipid peroxidation in activate cytochrome P-50. Exp Toxicol Pathol 1999; 51: 294–8.
Keller JN, Hanni KB, Markesberry WR. 4-Hydroxinonenal increases neuronal susceptibility to oxidative stress. J Neurosci Res 1999; 58: 823–30.
Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo 1999; 13: 295–309.
Grant MB, Wargovich TJ, Ellis EA, Cabellero BS, Mansour M, Pepine CJ. Localization of insulin-like growth factor I and inhibition of coronary smooth muscle cell growth by somatostatin analogues in human coronary smooth muscle cells. Circulation 1994; 89: 1511–7.
Grigorevski VP, Korotkina RN, Karelin AA. Effects of several regulatory peptides on the functional activity of the pancreas in acute experimental pancreatitis. Biull Eksp Biol Med 1989; 108: 428–30.
Szabo M, Droy-Lefaix MT, Doly M, Braquet P. Modification of ischemia-reperfusion induced ion shifts (Na, K, Ca and Mg) by free radical scavengers in the rat retina. Ophthalm Res 1993; 25: 1–9.
Hinder RA, Stein HJ. Oxygen derived free radicals. Arch Surg 1991; 126: 104–6.
Wiedermann CJ, Sitte B, Zilian U, Reinisch N, Beimpold H, Finkenstedt G, Braunsteiner H. Inhibition of superoxide anion release from circulating neutrophils by L-arginine in man. Clin Invest 1993; 71: 985–99
Szabo ME, Droy-Lefaix MT, Doly M, Carre C, Braquet P. Ischemia and reperfusion induced histological changes in rat retina. Invest Ophthalmol Vis Sci 1991; 32: 1471–8.
Wiedermann CJ, Niedermühlbichler M, Braunsteiner H. Inhibition of recombinant human growth hormone-induced and prolactin-induced activation of neutrophils by octreotide. Naunyn-Schmiedeberg's Arch Pharmacol 1992; 347: 336–41.
Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J. Increased serum nitrite and nitrate levels in patients with cirrhosis. Hepatology 1993; 18: 1139–42.
Chintila MS, Chiu PJS, Vermulapalli S, Watkins RW, Sybert EJ. Inhibition of EDRF aggravates ischemic acute renal failure in anesthetized rats. Naunyn Schmiedeberg's Arch Pharmacol 1993; 348: 305–10.
Pinsky DJ, Oz MC, Koga S. Cardiac preservation is enhanced in a heterotrophic rat transplant model by supplementing the nitric oxide pathway. J Clin Invest 1994; 93: 2291–7.
Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium derived relaxing factor. Nature 1986; 320: 454–6.
Davidge ST, Ojimba J, McLaughin MK. Vascular function in the vitamin E-deprived rat. An interaction between nitric oxide and superoxide anions. Hypertension 1998; 31: 830–5.
Köken T, Inal M. The effect of nitric oxide on ischemia-reperfusion injury in rat liver. Clin Chim Acta 1999; 288: 55–62.
Wenger FA, Klian M, Jacobi CA, Mauthsch I, Peter FJ, Guski H, Schimke I, Muller JM. Effects of octreotide on liver metastasis and intrametastatic lipid peroxidation in experimental pancreatic cancer. Oncology 2001; 60: 282–8.
De Belder AJ, Rodomski MW, Why HJF. Nitric oxide synthase activities in human myocardium. Lancet 1993; 341: 83–5.
McCall T, Broughton-Smith NK, Palmer RMJ, Whittle BJR, Mponcada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J 1989; 261: 293–6.
Kubes P, Suzuki M, Granger DN. Nitric oxide: An endogenous modulator of leukocytes adhesion. Proc Natl Acad Sci USA 1991; 88: 4651–5.
Suzuki M, Inauen W, Kvietya PR, Grisham MB, Meininger C, Schelling ME. Superoxide mediates reperfusion induced leukocyte-endothelial cell interactions. Am J Phsiol 1989; 257: 1740–5.
Weber M, Mohand S, Hicks D, Dreyfus H, Sahel JA. Monosialoganglioside GM1 reduces ischemia-reperfusion induced injury in rat retina. Invest Ophthalmol Vis Sci 1996; 37: 267–73.
Hughes WF. Quantification of ischemic damage in rat retina. Exp Eye Res 1991; 53: 573–82.
Adachi K, Fujita Y, Morizane C, Akaike A, Ueda M, Satoh M, Masai H, Kashii S, Honda Y. Inhibition of NMDA receptors and nitric oxide synthase reduces ischemic injury of the retina. Eur J Pharmacol 1998; 53–7.
Günal Aí, Isìk A, Celiker H, Eren O, Celebi H, Günal SY. Short term reduction of left ventricular mass in primary hypertrophic cardiomyopathy by octreotide injections. Heart 1996; 76: 418–21.
Toriumi Y, Samuel I, Wilcokson DP, Turkelson CM, Solomon TE, Joehl RJ. Octreotide and cholecystokinin reduce edema in obstruction induced acute pancreatitis. J Lab Clin Med 1993; 122: 450–4.
Author information
Authors and Affiliations
Additional information
This study was presented in part at the 23rd Congress of the European Society of Ophthalmology.
Rights and permissions
About this article
Cite this article
Celiker, U., Ilhan, N. Nitric oxide and octreotide in retinal ischemia–reperfusion injury* . Doc Ophthalmol 105, 327–338 (2002). https://doi.org/10.1023/A:1021243126512
Issue Date:
DOI: https://doi.org/10.1023/A:1021243126512