Abstract
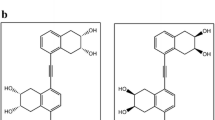
This paper reports the first study of an inclusion complex of abrassinosteroid with β-cyclodextrin. The formation of inclusion complexesbetween 24-epibrassinolide and β-cyclodextrin was confirmed by theirphysicochemical properties and the compounds were analysed by differentialscanning calorimetry, powder X-ray diffraction, nuclear magnetic resonancespectrometry and scanning electron microscopy. Theoretical calculations usingthe MM+ HyperChem force field showed a preference for inclusion of thesidechain of the epibrassinolide molecule into the β-cyclodextrin cavity toform a 1:1 inclusion complex, although complexes involving inclusion ofthe steroidal nucleus also possess a favourable interaction energy. Rice laminainclination assay, employing IAC-103 and IAC-104 cultivars, showed an improvedactivity for the epibrassinolide-cyclodextrin complex compared to theepibrassinolide itself. The results suggest that brassinosteroid complexationwith cyclodextrins may enhance the biological activity of these plant growthregulators.
Similar content being viewed by others
References
Ahmed S.M. 1998. Improvement of solubility and dissolution of 19-norprogesterone via inclusion complexation. J. Inclusion Phenom. Mol. Rec. Chem. 30: 111–125.
Alberts E. and Muller B.W. 1992. Complexation of steroid-hormones with cyclodextrin derivatives. Substituent effects of the guest molecule on solubility and stability in aqueous-solution. J. Pharm. Sci. 81: 756–761.
Braun P. and Wild A. 1984. The influence of brassinosteroids on growth and parameters of photosynthesis of wheat and mustard plants. J. Plant Physiol. 116: 189–196.
Brutti C., Apostolo N.M., Ferrerotti S.A., Llorente B.E. and Krymkiewicz N. 2000. Micropropagation of Cynara scolymus L. employing cyclodextrins to promote rhizogenesis. Sci. Hortic. 83: 1–10.
Clouse S.D. and Sasse J.M. 1998. Brassinosteroids: essential regulators of plant growth and development. Annual Rev. Plant Physiol. Plant Molec. Biol. 49: 427–451.
Connors A. 1997. The stability of cyclodextrin complexes in solution. Chem. Rev. 97: 1325–1357.
Cutler H.G., Yokota T. and Adam G. (eds) 1991. Brassinosteroids: Chemistry, Bioactivity and Applications. ACS Symposium Series 474. American Chemical Society, Washington, DC.
De Azevedo M.B.A., Alderete J.B., Lino A.C.S., Loh W., Faljoni-Alario A. and Durán N. 2000. Violacein/β-cyclodextrin inclusion complex formation studied by measurements of diffusion coefficient and circular dichroism. J. Incl. Phenom. Macrocyclic Chem. 37: 67–74.
De Azevedo M.B.M., Alderete J., Zullo M.A.T., Salva T.J.G. and Duran N. 2000. Brassinosteroids: a new class of plant hormones. The biological activity of 24-epibrassinolide and an inclusion complex of 24-epibrassinolide and β-cyclodextrin. Proceed. Int'l. Control. Rel. Bioact. Mater. Controlled Release Society, Inc.: 5006–5007.
Durán N., De Azevedo M.B.M., Zullo M.A.T., Salva T.J.G. and Alderete J.B. 2000. Process of cyclodextrin/brassinosteroids formulation, for agricultural application, used as plant hormones, Brazilian Patent BR9906202-A.
Durzan D.J. and Ventimiglia F.F. 2000. Cyclodextrin nutrients in plant tissue cultures, US Patent US 6087176 [Chem. Abstr. 133: 88976 (2000)].
Fujioka S., Noguchi T., Takatsuto S. and Yoshida S. 1998. Activity of brassinosteroids in the dwarf rice lamina inclination bioassay. Phytochemistry 49: 1841–1848.
Fujioka S. and Sakurai A. 1997. Biosynthesis and metabolism of brassinosteroids. Physiol. Plant. 100: 710–715.
Gosset S. and Gauvrit C. 1992. Activity enhancement of benzamide herbicides by cyclodextrins. French 9222204 A1 [Chem. Abstr. 118: 75387 (1997)].
Hayashi T., Iijima Y., Hoshino A. and Nakamura M. 1998. Agents and method for flowering acceleration using cinnamic acid-cyclodextrin inclusion compounds Japanese Patent. Kokai Tokkyo Koho JP 10273404 A2 [Chem. Abstr. 129: 327304 (1999)].
Huet H. and Jullien M. 1992. The β-cyclodextrins delay the germination of the somatic embryos of carrot (Daucus carota L.). Acad. Sci., Ser. III 314: 171–177.
Ikekawa N. and Zhao Y. 1991. Application of 24-epibrassinolide in agriculture. In: Cutler H.G., Yokota T. and Adam G. (eds), Brassinosteroids: Chemistry, Bioactivity and Applications. ACS Symposium Series 474. American Chemical Society, Washington, DC, pp. 280–305.
Kalinch F.N., Mandava N.B. and Todhunter J.A. 1985. Relationship of nucleic acid metabolism to brassinolide-induced responses in bean. J. Plant Physiol. 120: 207–214.
Khripach V.A., Zhabinskii V.N. and De Groot A.E. 1999. Brassinosteroids, a new class of plant hormones. Academic Press, Harcourt Brace &; Company, USA.
Koehler G., Grabner G., Klein C.T.H., Marconi G., Mayer B., Monti S. et al. 1996. Structure spectroscopic properties of cyclodextrin inclusion complexes. J. Inc. Phenom. Mol. Rec. Chem. 25: 103–108.
Lipkowitz K.B. 1998. Applications of computational chemistry to the study of cyclodextrins. Chem. Rev. 98: 1829–1873.
Mandava N.B. 1988. Plant growth promoting brassinosteroids. Ann. Rev. Plant Physiol. Plant Mol. Biol. 39: 23–52.
Marquart V. and Adam G. 1991. Recent advances in brassinosteroids research. In: Ebing W. (ed.), Chemistry of Plant Protection, Herbicide Resistance-Brassinosteroids, Gibberellins, Plant Growth Regulators. Vol. 7. Springer-Verlag, Berlin, pp. 104–139.
Marzona M., Carpignano R. and Quagliotto P. 1992. Quantitative structure-stability relationships in the inclusion complexes of steroids with cyclodextrins. Annal. Di Chim. 82: 517–537.
Okii M. 1993. Cyclodextrins for enhanced callus tissue culture of rice Japanese Patent. Kokai Tokkyo Koho JP 05292955 A2 [Chem. Abstr. 120: 1324504 (1997)].
Rajagopalan N., Chen S.C. and Chow W.S. 1986. A study of the inclusion complex of amphotericin-B with cyclodextrin. Int. J. Pharm. 29: 161–168.
Sairam P.K. 1994. Effect of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture stress conditions of two wheat varieties. Plant Growth Regul. 14: 173–181.
Sakurai A. and Fujioka S. 1993. The current status of physiology and biochemistry of brassinosteroids. Plant Growth Regul. 13: 147–159.
Sasse J.M. 1997. Recent progress in brassinosteroid research. Physiol. Plant. 100: 696–701.
Sawamoto T. 2000. Plant growth-stimulating and disease-preventing agents containing 1-triacontanol and their manufacture Japanese Patent 2000128707 A2 [Chem. Abstr. 132: 304657].
Stella V.J. and Rajewski R.A. 1997. Cyclodextrins: their future in drug formulation and delivery. Pharm. Res. 14: 556–567.
Stella V.J., Rao V.M., Zannou E.A. and Zia V. 1999. Mechanisms of drug release from cyclodextrin complexes. Adv. Drugs Del. Rev. 36: 3–16.
Szejtli J., Szente L., Harshegyi J., Daroczi I., Vorashazy L. and Torok S. 1989. Inclusion complexes and mixtures of plant growth regulators with cyclodextrins., Hung. Patent Teljes HU 47961 A2 [Chemical Abstracts 112: 50398 (1997)].
Takeno K. and Pharis R.P. 1982. Brassinosteroid-induced bending of the leaf lamina of dwarf rice seedlings: an auxin-mediated phenomenon. Plant Cell Physiol. 23: 1275–1281.
Uden W.V. and Woerdenbag H.J. 1994. Cyclodextrins as a useful tool for bioconversions in plant cell biotechnology. Plant Cell Tissue Organ Cult. 38: 103–113.
Vardhini B.V. and Rao S.S.R. 1999. Effect of brassinosteroids on nodulation and nitrogenase activity in groundnut Arachis hypogaea L. Phytochemistry 48: 927–930.
Wada K., Marumo S., Abe H., Morishita T., Nakamura K., Uchiyama M. et al. 1984. A rice lamina inclination test-a micro-quantitative bioassay for brassinosteroids. Agric. Biol. Chem. 48: 719–726.
Wada K., Marumo S., Ikekawa N., Morisaki M. and Mori K. 1981. Brassinolide and homobrassinolide promotion of lamina inclination of rice seedlings. Plant Cell Physiol. 22: 323–326.
Zhou D., Wu Y., Xu Q., Yang L., Bai C. and Tan Z. 2000. Molecular mechanics study of the inclusion of trimethylbenzene isomers in α-cyclodextrin. J. Inc. Phenom. Macrocyclic Chem. 37: 273–279.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Azevedo, M., Zullo, M., Alderete, J. et al. Characterisation and properties of the inclusion complex of 24-epibrassinolide with β-cyclodextrin. Plant Growth Regulation 37, 233–240 (2002). https://doi.org/10.1023/A:1020842727497
Issue Date:
DOI: https://doi.org/10.1023/A:1020842727497