Abstract
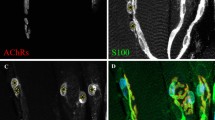
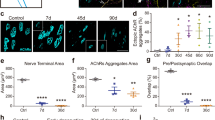
The neuromuscular junctions (NMJs) of postnatal rat soleus muscles were examined by immunohistochemical staining for S100, a marker of Schwann cells (SCs), and for protein gene product 9.5, a neuronal marker, to elucidate the involvement of SCs in synapse elimination. The morphological maturation of S100-immunoreactive terminal SCs at NMJs proceeded with the gradual increase in their number. The number of terminal SCs per NMJ was one or two at postnatal day (P) 7, reaching the adult number at P28, when it became three or four. Confocal laser scanning microscopic analysis of multi-innervated NMJs, whose number decreased between P7 and P14, revealed a change in the ratio between terminal SCs and axons with age. At P7, the ratio between axons and terminal SCs per NMJ was ≥2:1, which was exactly the reverse of that in adults, while at P14 this had changed to 2:2. A structural change appeared to occur at the same time at the preterminal region, this being prior to the establishment of a 1:1 relationship between axon and SC sheath which was detected at P14, with the ≥2:1 relationship seeming to occur at P7. Thus, synapse elimination seems to proceed, at least for one week, with the gradual loss of axons which are at different stages of maturation with respect to their spatial relationship with SCs. From our results it seems unlikely that SCs play an active role in selecting a single axon to survive.
Similar content being viewed by others
References
Ann, E. S., Mizoguchi, A., Okajima, S. & Ide, C. (1994) Motor axon terminal regeneration as studied by protein gene product 9.5 immunohistochemistry in the rat. Archives of Histology and Cytology 57, 317–30.
Balice-Gordon, R., Chua, C. K., Nelson, C. C. & Lichtman, J. W. (1993) Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron 11, 801–15.
Bixby, J. L. (1981) Ultrastructral observations on synapse elimination in neonatal rabbit skeletal muscle. Journal of Neurocytology 10, 81–100.
Brockes, J. P., Fields, K. L. & Raff, M. C. (1977) A surface antigenic marker for rat Schwann cells. Nature 266, 364–6.
Brown, M. C., Jansen, J. K. S. & van Essen, D. (1976) Polyneuronal innervation of skeletal muscle in newborn rats and its elimination during maturation. Journal of Physiology 261, 387–422.
Brown, M. C. (1984) Sprouting of motor nerves in adult muscles: a recapitulation of ontogeny. Trends in Neurosciences 7, 10–14.
Chen, L. & Ko, C. P. (1994) Extension of synaptic extracellular matrix during nerve terminal sprouting in living frog neuromuscular junctions. Journal ofNeuroscience 14, 796–808.
Colman, H. & Lichtman, J. W. (1993) Interactions between nerve and muscle: Synapse elimination at the developing neuromuscular junction. Developmental Biology 156, 1–10.
Colman, H., Nabekura, J. & Lichtman, J. W. (1997) Alterations in synaptic strength preceding axon withdrawal. Science 275, 356–61.
Georgiou, J., Robitaille, R., Trimble, W. S. & Charlton, M. P. (1994) Synaptic regulation of glial protein expression in vivo. Neuron 12, 443–55.
Jackson, S. G. & Suter, U. (1994) Signaling pathways mediating axon-Schwann cell interactions. Trends in Neurosciences 17, 399–401.
Jessen, K. R. & Mirsky, R. (1991) Schwann cell precursors and their development. Glia 4, 185–94.
Keynes, R. J. (1987) Schwann cells during neural development and regeneration: leaders or followers? Trends in Neurosciences 10, 137–9.
Korneliussen, H. & Jansen, J. K. S. (1976) Morphological aspects of the elimination of polyneuronal innervation of skeletal muscle fibers in newborn rats. Journal of Neurocytology 5, 591–604.
Martini, R. (1994) Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. Journal of Neurocytology 23, 1–28.
O' Brien, R. A. D., Ostberg, A. J. C. & Vrbova, G. (1978) Observations of the elimination of polyneuronal innervation in developing mammalian skeletal muscle. Journal of Physiology 282, 571–82.
Ogata, T. & Yamasaki, Y. (1984) Scanning electron microscope studies on the Schwann cells in rat motor endplates with special reference to their finger-like projections. Archirum Histologicum Japonicum 47, 533–9.
Pestronk, A. & Drachman, D. B. (1978) A new stain for quantitative measurement of sprouting at neuromuscular junctions. Muscle and Nerve 1, 70–4.
Purves, D. & Lichtman, J. W. (1980) Elimination of synapses in the developing nervous system. Science 210, 153–7.
Reynolds, M. L. & Woolf, C. J. (1992) Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. Journal of Neurocytology 21, 50–66.
Riley, D. A. (1977) Spontaneous elimination of nerve terminals from the endplates of developing skeletal myofibres. Brain Research 134, 279–85.
Riley, D. A. (1981) Ultrastructural evidence for axon retraction during the spontaneous elimination of polyneuronal innervation of the rat soleus muscle. Journal of Neurocytology 10, 425–40.
Rosenthal, J. L. & Taraskevich, P. S. (1977) Reduction of multiaxonal innervation at the neuromuscular junction of the rat during development. Journal of Physiology 270, 299–310.
Slater, C. R. (1982) Postnatal maturation of nerve-muscle junctions in hind limb muscles of the mouse. Developmental Biology 94, 11–22.
Son, Y. J. & Thompson, W. J. (1995a) Schwann cell processes guide regeneration of peripheral axons. Neuron 14, 125–132.
Son, Y. J. & Thompson, W. J. (1995b) Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron 14, 133–41.
Stefansson, K., Wollmann, R. L. & Moore, B. W. (1982) Distribution of S100 protein outside the central nervous system. Brain Research 234, 309–17.
Steinbach, J. H. (1981) Developmental changes in acetylcholine receptor aggregates at rat skeletal neuromuscular junctions. Developmental Biology 84, 267–76.
Thompson, R. J., Doran, J. F., Jackson, P., Dhillon, A. P. & Rode, J. (1983) PGP 9.5-a new marker for vertebrate neurons and neuroendocrine cells. Brain Research 278, 224–8.
Trachtenberg, J. T. & Thompson, W. J. (1996) Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature 379, 174–77.
Volpe, P., Biral, D., Pizzo, P., Salviati, G. & Margreth, A. (1993) Ontogenesis of chick iris intrinsic muscles: Evidence for a smooth-to-striated muscle transition. Developmental Biology 159, 441–9.
Webster, H. Def, Martin, J. R. & O' Connell, M. F. (1973) The relationships between interphase Schwann cells and axons before myelination: A quantitative electron microscopic study. Developmental Biology 32, 401–16.
Wilkinson, K. D., Lee, K., Deshpande, S., Duerksen-Hughes, P., Boss, J. M. & Pohl, J. (1989) The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl terminal hydrolase. Science 246, 670–3.
Woolf, C. J., Reynolds, M. L., Chong, M. S., Emson, P., Irwin, N. & Benowitz, L. I. (1992) Denervation of the motor endplate results in the rapid expression by terminal Schwann cells of the Growth-associated Protein GAP-43. Journal of Neuroscience 12, 3999–4010.
Ziskind-Conhaim, L. (1988) Physiological and morphological changes in developing peripheral nerves of rat embryos. Developmental Brain Research 42, 15–28.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirata, K., Zhou, C., Nakamura, K. et al. Postnatal development of Schwann cells at neuromuscular junctions, with special reference to synapse elimination. J Neurocytol 26, 799–809 (1997). https://doi.org/10.1023/A:1018570500052
Issue Date:
DOI: https://doi.org/10.1023/A:1018570500052