Abstract
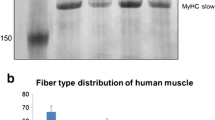
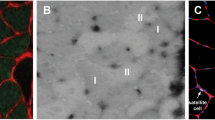
The main goal of this study was to find out, whether the appearance of fibres without evident myosin heavy chain (MyHC) transcript expression (negative fibres) implies the existence of additional MyHC transcripts in human muscle fibres. Fourteen different skeletal muscles were analysed also to verify how MyHC transcript expression matches histochemical phenotypes of fibres. For this purpose, the expression of β-slow, 2a and 2x MyHC transcripts, demonstrated by in situ hybridisation technique, was analysed within type I, IIC, IIA, IIAX and IIX fibres, determined according to the activity of myofibrillar ATPase. Additionally, MyHC isoform expression was immunohistochemically demonstrated and metabolic profiles of negative fibres were estimated. From a total of 4444 muscle fibres analysed, only 0.8% of fibres were negative, among them type I prevailed, the remainder were type IIA and IIX fibres. The majority of fibres expressed only β, 2a and 2x MyHC transcripts and they mostly matched type I, IIA and IIX fibres respectively, but two minor hybrid fibre groups (β/2a and 2ax) exhibited variable histochemical phenotype. The infrequency, the prevailing oxidative–glycolytic metabolic profile of negative type I fibres and frequent co-appearance with transitional type IIC fibres imply that the negative fibres rather result from fibre type transition than express an additional slow or even 2b MyHC transcripts. The appearance of hybrid and mismatched fibres additionally indicates that fibre type transition occurs also in presumably normal skeletal muscles, what enables the muscles to tune even with minimal changes in mechanical demands.
Similar content being viewed by others
References
Andersen LJ and Schiaffino S (1997) Mismatch between myosin heavy chain mRNA and protein distribution in human skeletal muscle fibres. Am J Physiol 272: C1881–C1889.
Andersen JL, Gruschy-Knudsen T, Sandri C, Larsson L and Schiaffino S (1999) Bed rest increases the amount of mismatched fibers in human skeletal muscle. J Appl Physiol 86(2): 455–460.
Bamman MM, Clarke MSF, Feeback DL, Talmadge RJ, Stevence BR, Lieberman SA and Greenisen MC (1998) Impact of resistance excercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 84(1): 157–163.
Biral D, Betto R, Danieli-Betto D and Salviati G (1988) Myosin heavy chain composition of single fibres from normal human muscle. Biochem J 250: 307–308.
Bredman JJ, Wessels A, Weijs WA, Korfage JAM, Soffers CAS and Moorman AF (1991) Demonstration of ‘cardiac-specific’ myosin heavy chain in masticatory muscles of human and rabbit. Histochem J 23: 160–170.
Brooke MH and Kaiser KK (1970) Muscle fiber types: how many and what kind? Arch Neurol 23: 369–379.
Cox RD and Buckingham M (1992) Actin and myosin genes are transcriptionally regulated during mouse skeletal muscle development. Dev Biol 149: 228–234.
Ennion S, Sant'ana Pereira J, Sargeant AJ, Young A and Goldspink G (1995) Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. J Muscle Res Cell Motil 16: 35–43.
Eržen I, Cvetko E, Obreza S and Angaut-Petit D (2000) Fiber types in the mouse levator auris longus muscle: a convinient preparation to study muscle and nerve plasticity. J Neurosci Res 59: 692–697.
Galler S, Hilber K and Pette D (1997) Strech activation and myosin heavy chain isoforms of rat, rabbit and human skeletal muscle fibres. J Muscle Res Cell Motil 18: 441–448.
Goldspink G, Scutt A, Loughna PT, Wells DJ, Jaenicke T and Gerlach GF (1992) Gene expression in skeletal muscle in response to strech and force generation. Am J Physiol 262: R356–R363.
Gorza L (1990) Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem 38: 257–265.
Guth L and Samaha FJ (1969) Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol 25: 138–152.
Harridge SDR, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjörnsson M and Saltin B (1996) Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflügers Arch-Eur J Physiol 432: 913–920.
Horton MJ, Brandon CA, Morris TJ, Yaw KM and Sciote JJ (2001) Expression of myosin heavy chain IIb RNA in human muscle. XXX European Muscle Conference, Pavia, Italy, September 2001, Abstract Book: 23.
Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA and Blau HM (1993) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol 158: 183–199.
Jandreski MA, Sole MJ and Liew CC (1987) Two different forms of beta myosin heavy chain are expressed in human striated muscle. Hum Genet 77: 127–131.
Jasmin BJ, Boudreau-Larivière C, Chan R, Hubatsch DA and Sveistrup H (1995) Neuromuscular factors in.uencing acetylcholinesterase gene expression in skeletal muscle fibres. In: Quinn DM, Balasubramanian AS, Doctor BP & Taylor P (eds) Enzymes of the Cholinesterase Family. (pp. 261–267) Plenum Press, New York.
Karsch-Mizrachi I, Feghali R, Shows TB and Leinwand LA (1990) Generation of full-length human perinatal myosin heavy-chain-encoding cDNA. Gene 89: 289–294.
Karsch-Mizrachi I, Travis M, Blau E and Leinwand LA (1989) Expression and DNA sequence analysis of a human embryonic skeletal muscle myosin heavy chain gene. Nucleic Acids Res 17: 6167–6179.
Kugler P (1991) Microphotometric determination of enzymes in brain sections. V. Glycerophosphate dehydrogenases. Histochemistry 95: 579–583.
Larsson L and Moss RL (1993) Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 472: 595–614.
Lefaucheur L, Hoffman RK, Gerrard DE, Okamura CS, Rubinstein N and Kelly A (1998) Evidence for three adult fast myosin heavy chain isoforms in type II skeletal muscle fibers in pigs. J Anim Sci 76: 1584–1593.
Leinwand LA, Fournier RE, Nadal-Ginard B and Shows TB (1983) Multigene family for sarcomeric myosin heavy chain in mouse and human DNA: localization on a single chromosome. Science 221: 766–769.
Lomprè AM, Nadal-Ginard B and Mahdavi V (1984) Expression of the cardiac ventricular α-and β-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem 259: 6437–6446.
Monemi M, Eriksson PO, Kadi F, Butler-Browne GS and Thornell LE (1999) Opposite changes in myosin heavy chain composition of human masseter and biceps brachii muscles during aging. J Muscle Res Cell Motil 20: 351–361.
Novikoff AB, Shin WY and Drucker J (1961) Mitochondrial localization of oxidative enzymes: staining results with two tetrasolium salts. J Biochem Cytol 9: 47–62.
Padykula HA and Herman E (1955) The specificity of the histochemical method foradenosine triphosphate. J Histochem Cytochem 3: 170–183.
Pette D and Vrbova G (1989) Neural control of phenotypic expression in mammalian muscle fibres. Muscle Nerve 8: 676–689.
Peuker H, Conjard A, Putman CT and Pette D (1999) Transient expression of myosin heavy chain MHCIα in rabbit muscle during fast-to-slow transition. J Muscle Res Cell Motil 20: 147–154.
Pierobon-Bormioli S, Sartore S, Dalla Libera L, Vitadello M and Schiaffino S (1981) ‘Fast’ isomyosins and fibertypes in mammalian skeletal muscle. J Histochem Cytochem 29: 1179–1188.
Russel B and Dix DJ (1992) Mechanisms for intracellular distribution of mRNA: in situ hybridization studies in muscle. Am J Physiol 262: C1–C8.
Saez LJ, Gianola KM, McNally EM, Feghali R, Eddy R, Shows TB and Leinwand LA (1987) Human cardiac myosin heavy chain genes and their linkage in the genome. Nucleic Acids Res 15: 5443–5459.
Sant’Ana Pereira JA, Ennion S, Sargeant AJ, Moorman AF and Goldspink G (1997) Comparison of the molecular, antigenic and ATPase determinants of fast myosin heavy chains in rat and human: a single fibre study. Eur J Physiol 435: 151–163.
Sant’Ana Pereira JA, Wessels A, Nijtmans L, Moorman AF and Sargeant AJ (1995) New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid fibre types. J Muscle Res Cell Motil 16: 21–34.
Sassoon D, Garner I and Buckingham M (1988) Transcripts of α-cardiac and α-skeletal actins are early markers for myogenesis in the mouse embryo. Development (Camb.) 104: 155–164.
Schiaffino S and Reggiani C (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiological Review 76: 371–423.
Schiaffino S, Gorza L, Sartore S, Saggin L, Vianello M, Gundersen K and Lomo T (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10: 197–205.
Sciote JJ, Rowlerson AM, Hopper C and Hunt NP (1994) Fibre type classification and myosin isoforms in human masseter muscle. J Neurol Sci 126: 15–24.
Serrano AL, Perez M, Lucia A, Chicharro JL, Quiroz-Rothe E and Rivero JLL (2001) Immunolabelling, histochemistry and in situ hybridisation in human skeletal muscle fibres to detect myosin heavy chain expression at the protein and mRNA level. J Anat 199: 329–337.
Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand LA and Schiaffino S (1994) Type IIx myosin heavy chain transcripts are expressed in type IIX fibers of human skeletal muscle. Am J Physiol 267: C1723–1728.
Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand LA and Schiaffino S (1995) Fibre type specific and developmentally regulated expression of five myosin heavy chain genes in human skeletal muscle. J Muscle Res Cell Motil 16: 176.
Staron RS (1991) Correlation between myofibrillar ATPase activity and myosin heavy chain composition in single human muscle fibers. Histochemistry 96: 21–24.
Talmadge RL (2000) Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve 23: 661–679.
Weiss A, McDonough D, Wertman B, Acakpo-Satchivi L, Montgomery K, Kucherlapati R, Leinwand LA and Krauter K (1999a) Organization of human and mouse skeletal myosin heavy chain gene clusters is highly conserved. Proc Natl Acad Sci USA 96: 2958–2963.
Weiss A, Schiaffino S and Leinwand LA (1999b) Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implication for functional diversity. J Mol Biol 290: 61–75.
Welle S, Bhatt K and Thornton CA (1999) Stimulation of myofibrillar synthesis by excercise is mediated by more efficient translation of mRNA. J Appl Physiol 86(4): 1220–1225.
Yoon SJ, Seiler SH, Kucherlapati R and Leinwand LA (1992) Organization of the human skeletal myosin heavy chain gene cluster. Proc Natl Acad Sci USA 89: 12078–12082.
Zhao WP, Kawaguchi Y, Matsui H, Kanamori M and Kimura T (2000) Histochemistry and morphology of the multifidus muscle in lumbar disc herniation. Comparative study between diseased and normal sides. Spine 25(17): 2191–2199.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smerdu, V., Eržen, I. Dynamic nature of fibre-type specific expression of myosin heavy chain transcripts in 14 different human skeletal muscles. J Muscle Res Cell Motil 22, 647–655 (2001). https://doi.org/10.1023/A:1016337806308
Issue Date:
DOI: https://doi.org/10.1023/A:1016337806308