Abstract
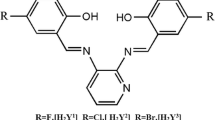
Fulleropyrrolidine containing a sterically hindered phenolic fragment was synthesized by the reaction of fullerene C60 with N-methylglycine and 3,5-di-tert-butyl-4-hydroxybenzaldehyde. Electrochemical reduction of fulleropyrrolidine-containing phenol 1 and the corresponding phenoxide ion proceeded stepwise to form stable radical anions, dianions, and trianions. The radical anion (g = 2.0000) and the phenoxyl radical (g = 2.0045) obtained by chemical oxidation with lead dioxide were identified by ESR spectroscopy. The electron affinity of fulleropyrrolidine was estimated at 2.58 eV. For the phenoxide ion, the electrochemical gap was determined (ΔE = E I ox – E I red = 0.47 V). The heats of formation and the energies of the frontier orbitals of fulleropyrrolidine and its transformation products were evaluated by the PM3 method.
Similar content being viewed by others
References
W. Kraetschmer, L. D. Lamb, K. Fostiropoulos, and D. R. Huffman, Nature, 1990, 347, 354.
E. Nakamura, H. Tokuyama, S. Yamago, T. Shiraki, and Y. Suigura, Bull. Chem. Soc. Jpn., 1996, 69, 2143.
T. Da Ros and M. Prato, Chem. Commun., 1999, 663.
D. V. Konarev and R. M. Lyubovskaya, Usp. Khim., 1999, 1, 23 [Russ. Chem. Rev., 1999, 1, 23 (Engl. Transl.)].
Y. Sun, T. Drovetskaya, R. D. Bolskar, R. Bau, P. D. W. Boyd, and C. A. Reed, J. Org. Chem., 1997, 62, 3642.
V. Brezova, A. Stasko, P. Rapta, D. M. Guldi, K.-D. Asmus, and K.-P. Dinse, Magn. Res. Chem., 1997, 35, 795.
N. M. Emanuel', Kinetika eksperimental'nykh opukholevykh protsessov [Kinetics of Experimental Tumor Processes], Nauka, Moscow, 1977, 184 pp. (in Russian).
L. D. Protsenko and Z. P. Bulkina, Khimiya i farmakologiya sinteticheskikh protivoopukholevykh preparatov, Spravochnik [Chemistry and Pharmacology of Synthetic Antitumor Medicines. Handbook], Naukova Dumka, Kiev, 1985, 246 pp. (in Russian).
M. Prato, M. Maggini, C. Giacometti, G. Scorrano, G. Sandona, and G. Farnia, Tetrahedron, 1996, 52, 5221.
M. Prato and M. Maggini, Acc. Chem. Res., 1998, 31, 519.
H. W. Kroto, A. W. Allaf, and S. P. Balm, Chem. Rev., 1991, 91, 1213.
V. D. Pokhodenko, Fenoksil'nye radikaly [Phenoxyl Radicals], Naukova Dumka, Kiev, 1969, 118 pp. (in Russian).
V. D. Pokhodenko, L. S. Degtyarev, V. G. Koshechko, and V. S. Kuts, Problemy khimii svobodnykh radikalov [Problems of Chemistry of Free Radicals], Naukova Dumka, Kiev, 1984, 264 pp. (in Russian).
A. L. Buchachenko and A. M. Vasserman, Stabil'nye radikaly [Stable Radicals], Khimiya, Moscow, 1973, 408 pp. (in Russian).
C. K. Mann and K. K. Barnes, Electrochemical Reactions in Nonaqueous Systems, Dekker, New York, 1970.
V. V. Ershov, G. A. Nikiforov, and A. A. Volod'kin, Prostranstvenno-zatrudnennye fenoly [Sterically Hindered Phenols], Khimiya, Moscow, 1972, 328 (in Russian).
V. V. Zverev, B. M. Musin, and V. V. Yanilkin, Zh. Obshch. Khim., 1997, 67, 1337 [Russ. J. Gen. Chem., 1997, 67 (Engl. Transl.)].
L. L. Muller, G. D. Nordblom, and E. A. Hayada, J. Org. Chem., 1972, 37, 916.
K. Mochida, A. Itani, M. Yokoyama, T. Tsuchiya, S. Worley, and J. Kochi, Bull. Chem. Soc. Jpn., 1985, 58, 2149.
V. V. Yanilkin and V. V. Zverev, Izv. Akad. Nauk, Ser. Khim., 1999, 682 [Russ. Chem. Bull., 1999, 48, 677 (Engl. Transl.)].
R. Kessinger, J. Crassons, A. Herrmann, M. Ruttimann, L. Echengoyen, and F. Diederich, Angew. Chem, 1998, 110, 2022.
I. A. Nuretdinov, V. V. Yanilkin, V. P. Gubskaya, N. I. Maksimyuk, and L. Sh. Berezhnaya, Izv. Akad. Nauk, Ser. Khim., 2000, 426 [Russ. Chem. Bull., Int. Ed., 2000, 49, 427].
M. Iyoda, F. Sultana, A. Kato, M. Yoshida, Y. Kuwatani, M. Komatsu, and S. Nagose, Chem. Lett., 1997, 63.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Kosoki, N. Matsunaga, K. N. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and S. A. Montgomery, J. Comput. Chem., 1993, 14, 1347.
J. J. P. Stewart, J. Comput. Chem, 1989, 10, 209.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nuretdinov, I.A., Gubskaya, V.P., Yanilkin, V.V. et al. Fulleropyrrolidine-containing sterically hindered phenol. Synthesis, structure, and properties. Russian Chemical Bulletin 50, 607–613 (2001). https://doi.org/10.1023/A:1011392207814
Issue Date:
DOI: https://doi.org/10.1023/A:1011392207814