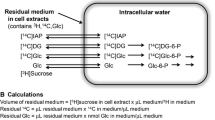
Abstract
Intracellular compartmentation of inositol was demonstrated in primary cultures of mouse astrocytes, incubated in isotonic medium, by determination of efflux kinetics after “loading” with [3H]inositol. Three kinetically different compartments were delineated. The largest and most slowly exchanging compartment had a halflife of ∼9 hr. This slow release leads to retention of a sizeable amount of pre-accumulated inositol in the tissue 24 hr after the onset of uptake inhibition, as confirmed by the observation that the inositol uptake inhibitor fucose caused a larger inhibition of unidirectional inositol uptake than of inositol pool size, measured as accumulated [3H]inositol after 24 hr of combined exposure to the inhibitor and the labeled isotope. Based upon the present observations and literature data, it is suggested that the large, slowly exchanging compartment is largely membrane-associated and participating in signaling via the phosphatidylinositide second messenger system, whereas inositol functioning as an osmolyte is distributed in the cytosol and located in one or both of the compartments showing a faster release.
Similar content being viewed by others
REFERENCES
Chen, Y. and Hertz, L. 1999. Noradrenaline effects on pyruvate decarboxylation-Correlation with calcium signaling. J. Neurosci. Res., 58:599–606.
Subbarao, K. V. and Hertz, L. 1990. Effects of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain Res. 527:346–349.
Subbarao, K. V. and Hertz, L. 1991. Stimulation of energy metabolism in astrocytes by adrenergic agonists. J. Neurosci. Res. 28:399–405.
Hansson, E. and Ronnback, L. 1988. Regulation of glutamate and GABA transport by adrenoceptors in primary astroglial cell cultures. Life Sci. 44:27–34.
Alexander, G. M., Grothuse, J. R., Gordon, S. W., and Schwartzman, R. J. 1997. Intracerebral microdialysis study of glutamate reuptake in awake, behaving rats. Brain Res. 766:1–10.
Chen, Y., Peng, L., Zhang, X., Stolzenburg, J.-U. and Hertz, L. 1995. Further evidence that fluoxetine interacts with a 5-HT1C receptor. Brain Res. Bull. 38:153–159.
Chen, Y., McNeill, J. R., Hajek, I., and Hertz, L. 1992. Effect of vasoprresin on brain swelling at the cellular level–Do astrocytes exhibit a furosemide-vasopressin-sensitive mechanism for volume regulation? Can. J. Physiol. Pharmacol. 70:S367-S373.
Chen, Y., Zhao, Z., and Hertz, L. Vasopressin effects on [Ca2+?]i and water permeability in differentiated astrocytes are mediated by potent activation of the V1b/V3 receptor, J. Neurosci. Res., in press.
Sarfaraz, D. and C. L. Fraser. 1999. Effects of arginine vasopressin on cell volume regulation in brain astrocytes in culture. Am. J. Physiol. 276 (3Pt. 1):E596-E601.
Brinton, R. D., Yamasaki, R., O'Neill, K., Gonzales, C., and Schreiber, S. 1998. Vasopressin induction of the immediate early response gene, NGFI-A in cultured hippocampal glial cells. Mol. Brain Res. 57:73–85.
Chen, Y. and Hertz, L. 1996. Inhibition of noradrenaline stimulated increase in [Ca2?]i in astrocytes by chronic treatment with a therapeutically relevant lithium concentration. Brain Res. 711:245–248.
Hallcher, L. M. and Sherman, W. R. 1980. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J. Biol. Chem. 255:10896–10901.
Berridge, M. J., Downes, C. P. and Hanley, M. R. 1989. Neural and developmental action of lithium: A unifying hypothesis. Cell 59:411–419.
Belmaker, R. H., Agam, G., van Calker, D., Richards, M. H., and Kofman, O. 1998. Behavioral reversal of lithium effects by four inositol isomers correlates perfectly with biochemical effects on the PI cycle; depletion by chronic lithium of brain inositol is specific to hypothalamus, and inositol levels may be abnormal in postmortem brain from bipolar patients. Neuropsychopharmacology 19:220–232.
Hertz, L., Wolfson M., Hertz, E., Agam, G., Richards M., and Belmaker, R. H. Lithium/inositol interactions. 1997. in Van Praag, H. M. and Honig A., eds. Depression: Neurobiological, Psychopathological and Therapeutic Advances. Wiley, New York, pp. 519–534.
Van Calker, D. and Belmaker, R. H. 1999. The high affinity inositol transport system-Implications for the pathophysiology and treatment of bipolar disorder. Bipolar Disorders, in press.
Brand, A., Richter-Landsberg, C. and Leibfritz, D. 1993. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 15:289–298.
Lubrich, B. and van Calker, D. 1999. Inhibition of the high-affinity myo-inositol uptake: A common mechanism of action of antibipolar drugs? Neuropsychopharmacology 21:519–529.
Wolfson, M., Bersudsky, Y., Zinger E., Simkin, M., Belmaker, R. H., and Hertz, L. 2000. Chronic treatment of human astrocytoma cells with lithium, carbamazepine or valproic acid decreases inositol uptake at high inositol concentrations, but increases it at low inositol concentrations. Brain Res. 855:158–161.
Glanville, N. T., Byers, D. M., Cook, H. V., Spence, M. W., and Palmer, F. B.StC. 1989. Differences in the metabolism of inositol and phoshoinositides by cultured cells of neuronal and glial origin. Biochim. Biophys. Acta 1004:69–179.
Einat, H., Kofman, O., Itkin, O., Lewitan, R. J., and Belmaker, R. H. 1998. Augmentation of lithium's behavioral effect by inositol uptake inhibitors. J. Neural Transmission 105:31–38.
Wolfson, M., Einat, H., Bersudsky, Y., Berkin, V., Belmaker, R. H., and Hertz, L. 1999. Nordidemnin potently inhibits inositol uptake in cultured astrocytes and dose-dependently augments lithium's proconvulsant effect in vivo, J. Neurosci. Res., 60:116–1221.
Honchar, M. P., Olney, J. W., and Sherman, W. R. 1983. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science 220:323–325.
Tricklebank, M. D., Singh, L., Jackson, A., and Oles, R. J. 1991. Evidence that a proconvulsant action of lithium is mediated by inhibition of myo-inositol phosphatase in mouse brain. Brain Res. 558:145–148.
Kofman, O., Sherman, W. R., Katz, V., and Belmaker, R. H. 1993. Restoration of brain myo-inositol levels in rats increases latency to lithium-pilocarpine seizures. Psychopharmacol. 110:229–234.
Bersudsky, Y., Shapiro, J., Agam, G., Kofman, O., and Belmaker, R. H. 1994. Behavioral evidence for the existance of two pools of cellular inositol. Eur Neuropsychopharmacol 4:463–467.
Isaacks, R. E., Bender, A. S., Kim, C. Y., Shi, Y. F., and Norenberg, M. D. 1999. Effect of ammonia and methionine sulfoximine on myo-inositol transport in cultured astrocytes. Neurochem. Res. 24:51–59.
Isaacks, R. E., Bender, A. S., Kim, C. Y, Shi, Y. F., and Norenberg, M. D. 1999. Effect of osmolality and anion channel inhibitors on myo-inositol efflux in cultured astrocytes. J. Neurosci. Res. 57: 866–871.
Yorek, M. A., Dunlap, J. A., Stefani, M. R., and Davidson, E. P. 1992. L-fucose is a potent inhibitor of myo-inositol transport and metabolism in cultured neuroblastoma cells. J. Neurochem. 58:1626–1636.
Batty, I. H., and Downes, C. P. 1995. The mechanism of muscarinic receptor-stimulated phosphatidylinositol resynthesis in 1321N1 astrocytoma cells and its inhibition by Li+. J. Neurochem. 65:2279–2289.
Wolfson, M., Hertz, E., Belmaker, R. H., and Hertz, L. 1998. Chronic treatment with lithium and pretreatment with excess inositol reduce inositol pool sizes by different mechanisms. Brain Res. 787:34–40.
Aukema, H. M. and Holub, B. J. 1994. Inositol and pyrroloquinoline quinone: A: Inositol. in Shils, E, Olson, J. A. and Shike, M. (eds.) Modern Nutrition in Health and Disease, 8th ed. Lea and Febiger, Philadelphia, pp. 466–473.
Agam, G., Balfour, N. Shapiro, H., and Belmaker, R. H. 1995. Plasma inositol levels in lithium-treated manic-depressives, schizophrenics and controls. Hum. Psychopharmacol. 10:311–314.
Hertz, L., Juurlink, B. H. J., and Szuchet, S. 1985. Cell cultures. in A. Lajtha, A. (ed.) Handbook of Neurochemistry, 2nd Ed., Vol. 8. Plenum Press, New York, pp. 603–661.
Juurlink, B. H. J., and Hertz, L. 1992. Astrocytes. in A. A. Boulton, A. A., Baker, G. B., and Walz, W. (eds.) Neuromethods: vol. 23, Cell Cultures, Humana Clifton, NY, pp. 269–321.
Hertz, L., Lai, J. C. K., and Peng, L. 1998. Functional studies in cultured astrocytes. Methods-A Companion to Methods in Enzymology 16:293–310.
Meier, E., Hertz, L., and Schousboe, A. 1991. Neurotransmitters as developmental signals. Neurochem. Int. 19:1–15.
Isaacks, R. E., Bender, A. S., Reuben, J. S., Kim, C. Y., Shi, Y. F., and Norenberg, M. D. 1999. Effect of dibutyryl cyclic AMP on the kinetics of myo-inositol transport in cultured astrocytes. J. Neurochem. 73:105–111.
Strange, K., Morrison, R., Heilig, C. W., DiPietro, S., and Gullians, S. R. 1991. Upregulation of inositol transport mediates inositol accumulation in hyperosmolar brain cells. Am. J. Physiol 263 (2Pt1):C412–419.
Batty, I. H. and Downes, C. P. 1994. The inhibition of phosphoinositide synthesis and muscarinic-receptor-mediated phospholipase C activity by Li??as secondary, selective, consequences of inositol depletion in 1321N1 cells. Biochem. J. 297:529–537.
Batty, I. H., Currie, R. A., and Downes, C. P. 1998. Evidence for a model of integrated inositol phospholipid pools implies an essential role for lipid transport in the maintenance of receptor-mediated phospholipase C activity in 1321N1 cells. Biochem. J. 330:1069–1077.
Jackson, P. S. and Strange, K. 1993. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am. J. Physiol. 265 (6Pt1):C1489-C1500.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wolfson, M., Bersudsky, Y., Hertz, E. et al. A Model of Inositol Compartmentation in Astrocytes Based Upon Efflux Kinetics and Slow Inositol Depletion after Uptake Inhibition. Neurochem Res 25, 977–982 (2000). https://doi.org/10.1023/A:1007556509371
Issue Date:
DOI: https://doi.org/10.1023/A:1007556509371