Abstract
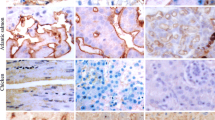
Sialic acid-containing glycoconjugates are generally considered to be unique to the deuterostomes, a lineage of the animal kingdom which includes animals from the echinoderms up to the vertebrates. There are, however, two isolated reports of sialic acid occurring in the insect species Drosophila melanogaster and Galleria mellonella. Since insects are classified as protostomes, these findings call previous assumption on the phylogenetic distribution and thus on the evolution of sialic acids into question. Here, we report the occurrence of N-acetylneuraminic acid (Neu5Ac) in larvae of the cicada Philaenus spumarius. Cytochemical analysis of larval sections with lectins from Sambucus nigra and Limax flavus suggested the presence of sialic acids in the concrement vacuoles of the Malpighian tubules. The monoclonal antibody MAb 735, which is specific for polysialic acid, labelled the same structures. A chemical analysis performed by HPLC of fluorescent derivatives of sialic acids and by GLC-MS provided sound evidence for the presence of Neu5Ac in the Philaenus spumarius larvae. These data suggest that in this cicada Neu5Ac occurs in α2,8-linked polysialic acid structures and in α2,6-linkages. The results provide further evidence for the existence of sialic acids in insects and in linkages known to occur in glycoconjugates of deuterostomate origin.
Similar content being viewed by others
References
Kelm S, Schauer R (1997) Sialic cids in molecular and cellular interactions. In Int. Rev. Cytology vol. 175 (Jeon KW, Jarnik JW, eds), Academic Press, San Diego, pp. 137–240.
Varki A (1992) Diversity in the sialic acids. Glycobiology 2: 25–40.
Warren L (1963) The distribution of sialic acids in nature. Comp Biochem Physiol 10: 153–71.
Corfield AP, Schauer R (1982) In Sialic AcidsChemistry, Metabolism and Function (Schauer R, ed) Cell Biology Monogr 10: pp 5–39. Wien/New York: Springer-Verlag.
Schauer R, Kamerling, JP (1997) Chemistry, biochemistry and biology of sialic acids. In Glycoproteins II, Vol. 29b (Montreuil J, Vliegenthart JFG, Schachter H, eds) pp 243–402. Amsterdam: Elsevier.
Schauer R, Vliegenthart JFG (1982) Introduction. In Sialic Acids, Chemistry, Metabolism and Function (Schauer R, ed) Cell Biology Monogr 10: pp 1–3. Wien/New York: Springer-Verlag.
Roggentin P, Schauer R, Hoyer LL, Vimr ER (1993) The sialidase superfamily and its spread by horizontal gene transfer. Mol Microbiol 9: 915–21.
Jarvis DL, Finn EE (1995) Biochemical analysis of the Nglycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology 212: 500–11
März L, Altmann F, Staudacher E, Kubelka V (1995) Protein glycosylation in insects. In Glycoproteins I, Vol. 29a (Montreuil J, Vliegenthart JFG, Schachter H, eds) pp 543–63. Amsterdam: Elsevier.
Altmann F (1997) More than silk and honey——or, can insect cells serve in the production of therapeutic glycoproteins? Glycoconjugate J 14: 643–6.
Lopez M, Tetaert D, Juliant S, Gazon M, Cerutti M, Verbert A, Delannoy P (1999) O-glycosylation potential of lepidopteran insect cell lines. Biochim Biophys Acta 1427: 49–61.
Hafer J, Ferenz H-J (1991) Locust vitellogenin receptor: an acidic glycoprotein with N-and O-linked oligosaccharides. Comp Biochem Physiol 100B: 579–86.
Rolle RS, Lawrence PO (1994) Characterisation of a 24 kD parasitism-specific hemolymph protein from pharate pupae of the Caribbean fruit fly Anastrepha suspensa. Arch Insect Biochem Physiol 25: 227–44.
Roth J, Kemp A, Reuter G. Schauer R, Gehring WJ (1992) Occurrence of sialic acids in Drosophila melanogaster. Science 256: 673–5.
Karaçali S, Kirmizigül S, Deveci R, Deveci Ö, Onat T, Gürcü B (1997) Presence of sialic acid in prothoracic glands of Galleria mellonella (Lepidoptera). Tissue & Cell 29: 315–21.
Frosch M, Görgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D (1985) NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: Isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci. USA 82: 1194–8.
Veh RW, Michalski JC, Corfield AP, Sander-Wewer M, Gies D, Schauer R (1981) A new chromatographic system for the rapid analysis and preparation of colostrum sialyloligosaccharides. J Chromatogr 212: 313–22.
Gerardy-Schahn R, Bethe A, Brennecke T, Möhlenhoff M, Eckhardt M, Ziesing S, Lottspeich F, Frosch M (1995) Molecular cloning and functional expression of bacteriophage PKIEencoded endoneuraminidase Endo NE. Mol Microbiol 16: 441–50.
Mawhinney TP, Chance DL (1994) Hydrolysis of sialic acids and O-acetylated sialic acids with propionic acid. Anal Biochem 223: 164–7.
Reuter G, Schauer R (1994) Determination of sialic acids. Methods Enzymol. 230: 168–99.
Malykh YN, Shaw L, Schauer R (1998) The role of CMP-Nacetylneuraminic acid hydroxylase in determining the level of Nglycolylneuraminic acid in porcine tissues. Glycoconjugate J 15: 885–93.
Reuter G, Schauer R (1994) Enzymic methods of sialic acid analysis. In Methods in Carbohydrate Chemistry, 10 (BeMiller JN, Manners DJ, Sturgeon RJ, eds) pp 29–39. New York: John Wiley & Sons.
Kamerling JP. Vliegenthart JFG (1982) Gas-liquid chromatography and mass spectrometry of sialic acids. In Sialic Acids Chemistry, Metabolism and Function (Schauer R, ed) Cell Biology Monogr 10: pp 95–125. Wien/New York: Springer-Verlag.
Wang WC, Cummings RD (1988) The immobilised leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked α2,3 to penultimate galactose residues. J Biol Chem 263: 4576–85.
Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WI (1987) The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Acα2,6Gal/GalNAc sequence. J Biol Chem 262: 1596–601.
Miller RL, Collawn JF Jr, Fish WW (1982) Purification and macromolecular properties of a sialic acid-specific lectin from the slug Limax flavus. J Biol Chem 257: 7574–80.
Seifert G (1995) In Entomologisches Praktikum (Seifert G, ed) pp 67–70. Stuttgart—New York: Georg Thieme Verlag.
Mello MLS (1979) A mucous secretion in the Malpighian tubes of a neotropical bumblebec, Bombus atratus Franklin. Protoplasma 99: 147–58.
Troy II FA (1995) Sialobiology and the polysialic acid glycotope: occurrence, structure, function, synthesis, and glycopathology. In Biology of the Sialic Acids (Rosenberg A, ed) pp 95–144. New York: Plenum Press.
Inoue S, Inoue Y (1997) Fish glycoproteins. In Glycoproteins II, Vol. 29b (Montreuil J. Vliegenthart JFG. Schachter H, eds) pp 143–72. Amsterdam: Elsevier.
Svoboda M, Przybylski M, Schreurs J, Miyajima A, Hogeland K, Deinzer M (1991) Mass spectrometric determination of glycosylation sites and oligosaccharide composition of insect-expressed mouse interleukin-3. J Chromatog 562: 403–19.
Davis TR, Wood HA (1995) Intrinsic glycosylation potentials of insect cell cultures and insect larvae. In Vitro Cell Dev Biol.-Animal 31: 659–63.
Licari PJ, Jarvis DL, Bailey JE (1993) Insect cell hosts for baculovirus expression vectors contain endogenous exoglycosidase activity. Biotechnol Prog 9: 146–52.
Amino R, Porto RM, Chammas R, Egami MI, Schenkman S (1998) Identification and characterization of a sialidase released by the salivary gland of the hematophagous insect Triatoma infestans. J Biol Chem 273: 24575–82.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Malykh, Y.N., Krisch, B., Gerardy-Schahn, R. et al. The presence of N-acetylneuraminic acid in Malpighian tubules of larvae of the cicada Philaenus spumarius. Glycoconj J 16, 731–739 (1999). https://doi.org/10.1023/A:1007115627708
Issue Date:
DOI: https://doi.org/10.1023/A:1007115627708