Abstract
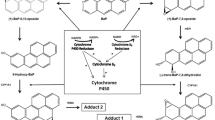
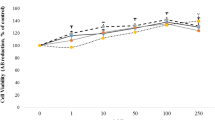
Treatment of intact C3H10T1/2 cells or microsomes therefrom with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and benzanthracene (BA) enhanced CYP1B1 activity and CYP1B1 expression as revealed by elevations of CYP1B1-catalyzed DMBA metabolism, CYP1B1 apoprotein level and CYP1B1 gene expression. One hundred µM DHEA caused an 80-90% inhibition of cellular DMBA metabolism without inflicting cell death. Cytosolic glucose-6-phosphate dehydrogenase (G6PDH) was also inhibited in DHEA-treated cells, presumably due to the inhibition of NADP reduction. In contrast, neither DMBA metabolism nor CYP1B1 apoprotein was inhibited by DHEA in the microsomes isolated from these cells. DHEA (100 μM), TCDD (10 nM) and BA (10 μM) stimulated the activities and increased the apoprotein levels of two peroxisomal enzymes, namely, acyl CoA oxidase (ACOX) and acyl CoA hydrolase (ACH2) and also induced the expression of CYP1B1 and ACOX genes. Cytosolic fatty acyl-CoA β-oxidation was also stimulated by DHEA, TCDD and BA. In corroboratory experiments, it was found that concomittant with the stimulation of the activity of a key enzyme regulator of fatty acid homeostasis, namely, glycerol-3-phosphate dehydrogenase (G3PDH), these agents enhanced arachidonic acid (AA) metabolism as judged by the release of [3H] from AA into the culture medium. Collectively, these data suggest that DHEA mediates the regulation of CYP1B1 and inhibits BA and TCDD-induced CYP1B1-catalyzed carcinogen (DMBA) activation in 10T1/2 cells through metabolic interactions that involve the activation of the peroxisomal and fatty acid β-oxidation signaling pathways. These results also present evidence for the first time, for the possible peroxisomal effects of TCDD and BA which are similar to those of DHEA in this mouse embryo fibroblast cell line.
Similar content being viewed by others
References
Savas U, Bhattacharya KK, Christou M, Alexander DL, Jefcoate CR: Mouse cytochrome P450EF, representative of a new 1B subfamily of cytochrome P-450s. J Biol Chem 269: 14905–14911, 1994
Bhattacharrya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR: Identificaton of rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. J Biol Chem 270: 11595–11602, 1995
Walker NJ, Gastel JA, Costa LT, Clark GC, Lucier GW, Sutter TR: Rat CYP1B1: An adrenal cytochrome P450 that exhibits sex-dependent expression in the livers and kidney of TCDD-treated animals. Carcinogenesis 16: 1319–1327, 1995
Pottenger LH, Jefcoate CR: Characterization of a novel cytochrome P450 from the transformable cell line, C3H/10T1/2. Carcinogenesis 11: 321–327, 1990
Savas U, Jefcoate CR: Dual regulation of P450EF expression via the aryl hydrocarbon receptor and protein stabilization in C3H/10T1/2 cells. Mol Pharmacol 23: 1153–1159, 1994
Shimada T, Hayes CL, Yamazaki SA, Hecht SS, Guengerich FP, Sutter TR: Activation of chemically diverse procarcinogens by human cytochrome P450 1B 1. Cancer Res 56: 2979–2984, 1996
Otto S, Marcus C, Pidgeon C, Jefcoate CR: A novel adrenocorticotropin-inducible cytochrome P450 from rat adrenal microsomes catalyzes polycyclic aromatic hydrocarbon metabolism. Endocrinol 129: 970–982, 1991
Otto S, Bhattacharrya KK, Jefcoate CR: Polycyclic aromatic hydrocarbon metabolism in rat adrenal, ovary and testis microsomes is catalyzed by the same novel cytochrome P450RAP. Endocrinol 131: 3067–3076, 1992
Savas U, Christou M, Jefcoate CR: Mouse endometrium stromal cells express a polycyclicaromatic hydrocarbon-inducible cytochrome P450 that closely resembles the novel P450 in mouse embryo fibroblasts. Carcinogenesis 14: 2013–2018, 1993
Christou M, Savas U, Schroeder S, Shen S, Thompson T, Gould M, Jefcoate CR: CYP1-A1 and CYP1B1 in the rat mammary gland: Cell specific expression and regulation by polycyclic aromatic hydrocarbons and hormones. Mol Cell Endocrinol 115: 50–58, 1995
Brake PB, Jefcoate CR: Regulation of cytochrome P451B1 in cultured rat adrenocortical cells by cyclic adenosine 3′,5′-monophosphate and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Endocrinol 136: 5034–5041, 1995
Li S, Yan X, Belanger A, Labrie F: Prevention by dehydroepiandrosterone of the development of mammary carcinoma induced by 7,12-dimethylbenzanthracene (DMBA) in the rat. Breast Cancer Res Treat 29: 203–217, 1994
Shibata MA, Hasegawa R, Imaida K, Hagiwara A, Ogawa K, Hirose M, Ito SS, Shirai T: Chemoprevention by dehydroepiandrosterone and indomethacin in a rat multiorgan carcinogenesis model. Cancer Res 55: 4870–4874, 1995
Casazza JP, Schaffer WT, Veech RL: The effect of dehydroepiandrosterone on liver metabolites. J Nutr 116: 304–310, 1986
Rao SM, Subbarao V, Yeldandi VA, Reddy JK: Hepatocarcinogenicity of dehydroepiandrosterone in the rat. Cancer Res 52: 2977–2979, 1992
Rao MS, Reddy JK: Peroxisome proliferation and hepatocarcinogenesis. Carcinogenesis 8: 631–636, 1987
Reddy J, Azamoff DL: Hypolipidemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature 283: 397–398, 1980
Portenger L, Christou M, Jefcoate CR: Characterization of a novel cytochrome from the transformable cell line C3H/10T1/2. Carcinogenesis 11: 321–327, 1991
Wu HQ, Masset BJ, Tweedie DJ, Milewich L, Frenkel RA, Martin WC, Estabrook RW, Prough RA: Induction of microsomal NADPH-cytochrome P450 reductase and cytochrome P4501VA by dehydroepiandrosterone in rats: A possible peroxisomal proliferator. Cancer Res 49: 2337–2343, 1989
Yamada JM, Sakuma T, Ikeda T, Fukuda K, Suga T: Characteristics of dehydroepiandrosterone as a peroxisomal proliferator. Biochem Biophys Acta 1092: 233–243, 1991
Rao MS, Reid B, Ide H, Subbarao V, Reddy JK: Dehydroepiandrosterone-induced peroxisomal proliferation in the rat: Evaluation of sex differences. Proc Soc Exp Biol Med 207: 186–190, 1994
Prough RA, Webb SJ, Wu HQ, Lapenson DP, Waxman DJ: Induction of microsomal and peroxisomal enzymes by dehydroepiandrosterone and its reduced metabolite in rats. Cancer Res 54: 2878–2886, 1994
Ram PA, Waxman DJ: Dehydroepiandrosterone 3 beta-sulfate is an endogenous activator of the peroxisome proliferation pathway: Induction of cytochrome P4504A and acyl-CoA oxidase mRNAs in primary rat hepatocyte culture and inhibitory effect of Ca2+ channel blockers. Biochem J 301: 753–758, 1994
Laemmli UK: Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227: 680–685, 1970
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Jones JT, Andrews SJ: Glucose-6-phosphate dehydrogenase activity in somatic and germinal cells of the mouse testis. J Repro Fert 54: 357–362, 1978
Kozak LP, Jensen JT: Genetic and developmental control of multiple forms of L-glycerol-3-phosphate dehydrogenase. J Biol Chem 249: 7775–7781, 1974
Lazarow PB: Assay of peroxisomal β-oxidation of fatty acids. Meth Enzymol 72: 315–319, 1981
Chu R, Varanasi U, Chu S, Lin Y, Usuda N, Rao SM, Reddy J: Overexpression and characterization of the human peroxisomal acyl-CoA oxidase in insect cells. J Biol Chem 270: 4908–4915, 1995
Yamada J, Matsumoto I, Furihata T, Sakuma M, Suga T: Purification and properties of long chain acyl-CoA hydrolases from the liver cytosol of rats treated with peroxisome proliferator. Arch Biochem Biophys 308: 118–125, 1994
Dell'Aquila ML, Herald CL, Kamano Y, Petit GR, Blumberg PM: Differential effects of bryostatins and phorbol esters on arachidonic acid metabolite release and epidermal growth factor binding in C3H10T1/2 cells. Cancer Res 48: 3702–3708, 1988
Ikegwuonu FI, Pai J-K, Mueller GC: Effects of steroids on the synthesis and metabolism of phosphatidylethanol in phorbol ester-activated lymphocytes. Carcinogenesis 11: 1927–1935, 1990
Inestrosa NC, Bronfman M, Leighton F: Detection of peroxisomal fatty acyl-CoA oxidase activity. Biochem J 182: 779–788, 1979
Leighton B, Tagliaferro AR, Newsholme EA: The effect of dehydroepiandrosterone acetate on liver peroxisomal enzyme activities of male and female rats. J Nutr 117: 1287–1290, 1987
Miyazawa S, Furuta S, Hashimoto T: Induction of a novel long chain acyl-CoA hydrolase in rat liver by administration of peroxisomal proliferators. Eur J Biochem 117: 424–430, 1981
Yamada J, Matsumoto I, Furihata T, Sakuma M, Suga T: Purification and properties of long chain acyl-CoA hydrolases from the liver cytosol of rats treated with peroxisome proliferator. Arch Biochem Biophys 308: 118–125, 1994
Kaikaus RM, Chan WK, Lysenko N, Ray R, Ortiz de Montellano PR, Bass NM: Induction of peroxisomal fatty acid β-oxidation and liver fatty acid-binding protein by peroxisome proliferators. J Biol Chem 268: 9593–9603, 1993
Lazarow PB, deDuve C: A fatty acyl-CoA oxidizing system in rat liver peroxisome: Enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci USA 73: 2043–2047, 1976
Su C-Y, Lardy H: Induction of hepatic mitochondrial glycerophosphate dehydrogenase in rats by dehydroepiandrosterone. J Biochem 110: 207–213, 1991
Marrero M, Prough RA, Frenkel RA, Milewitch L: Dehydroepiandrosterone feeding and protein phosphorylation, phosphatases and lipogenic enzymes in mouse liver. Proc Soc Exp Biol Med 193: 110–117, 1989
Thornton M, Moore MA, Ito N: Modifying influence of dehydroepiandrosterone or butylated hydroxytoluene treatment on initiation and development stage of azaserine-induced acinar pancreatic preneoplastic lesions in the rat. Carcinogenesis 10: 407–410, 1989
Loria RM, Padgett DA, Huynh PN: Regulation of the immune response by dehydroepiandrosterone and its metabolites. J Endocrinol 150: S209–S220, 1996
MacIndoe JH, Woods J, Jeffries L, Hinkhouse M: The hydrolysis of estrone sulfate dehydroepiandrosterone sulfate by MCF-7 human breast cancer cells. Endocrinol 123: 1281–1287, 1988
Bell DR, Elcombe CR: Induction of acyl-CoA oxidase and cytochrome P4501IVA RNA in rat primary hepatocyte culture by peroxisome proliferators. Biochem J 280: 249–253, 1991 100
Yamada J, Sakuma M, Suga T: Induction of peroxisomal β-oxidation enzymes by dehydroepiandrosterone and its sulfate in primary cultures of rat hepatocyte. Biochem Biophys Acta 1160: 231–236, 1992
Leighton B, Tagliaferro AR, Newsholme EA: The effect of dehydroepiandrosterone acetate on liver peroxisomal enzyme activities of male and female rats. J Nutr 117: 1287–1290, 1987
Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, Fuji-Kuriyama Y: Dioxin-binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem 269: 27337–27343, 1994
Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W: Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors Cell 68: 879–887, 1992
Isseman I, Green S: Activation of a member of the steroid hormone receptor superfamily by peroxisomal proliferators. Nature 347: 645–650, 1990
Peters JM, Zhou Y-C, Ram PA, Lee SST, Gonzalez FJ, Waxman DJ: Peroxisome proliferator-activated receptor α ?is required for gene induction by dehydroepiandrdsterone-3β-sulfate. Mol Pharmacol 50: 67–74, 1996
Gottlicher M, Widmark E, Li Q, Gustafsson JA: Fatty acids activate a chimera of the clofibric acid-activated receptor α? required for gene induction by dehydroepiandrosterone-3β-sulfate. Mol Pharmacol 50: 67–74, 1996
Zumoff B, Rosenfeld RS, Strain GW: Sex differences in the twenty four hour mean plasma concentrations of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) and the DHEA to DHEAS ratio in normal adults. J Clin Endocrinol Metab 51: 330–333, 1980
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ikegwuonu, F.I., Jefcoate, C.R. Evidence for the involvement of the fatty acid and peroxisomal β-oxidation pathways in the inhibition by dehydroepiandrosterone (DHEA) and induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and benz(a)anthracene (BA) of cytochrome P4501B1 (CYP1Bl) in mouse embryo fibroblasts (C3H10T1/2 cells). Mol Cell Biochem 198, 89–100 (1999). https://doi.org/10.1023/A:1006954216233
Issue Date:
DOI: https://doi.org/10.1023/A:1006954216233