Abstract
Colchicine has been demonstrated to suppress the release of fibroblast growth factors, retard collagen formation and augment collagenase activity. Trials with colchicine in patients with hepatic fibrosis have suggested clinical benefit. The development of impaired myocardial function in the spontaneously hypertensive rat (SHR) is associated with a marked increase in myocardial fibrosis. The present study was carried out to test the hypothesis that chronic colchicine administration to the SHR would prevent the development of fibrosis and impaired myocardial performance.
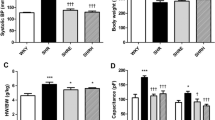
Colchicine (1 mg/l drinking water) was administered to male SHR and WKY rats from at age 13 months until 24 months or until evidence of heart failure was observed. Age-matched untreated SHR and colchicine treated and untreated WKY served as controls. At study, active and passive properties of isolated left ventricular muscle preparations were determined. Myocardial fibrosis was assessed by measuring hydroxyproline and histologic determination of interstitial cross-sectional area. Increases in LV hydroxyproline and interstitial area were found in untreated SHR relative to WKY; passive myocardial stiffness was increased and active muscle properties were depressed. In comparing colchicine treated vs untreated SHR, no differences in hydroxyproline, interstitial area or intrinsic myocardial function were found. In the WKY, colchicine increased myocardial interstitium and passive stiffness without changing hydroxyproline. Active myocardial function was not depressed.
Thus, chronic colchicine administration neither attenuated the development of interstitial fibrosis nor prevented impaired myocardial function in the SHR. Colchicine treatment was associated with increased interstitium in WKY with increased passive myocardial stiffness. (Mol Cell Biochem 166: 45-54, 1997)
Similar content being viewed by others
References
Inoue S: Effect of colchicine on microscopic and submicroscopic structure of the cardiac spindle. Exp Cell Res 2: 305–314, 1952
Taylor EW: The mechanism of colchicine inhibition of mitosis. J Cell Biol 25: 145–160, 1965
Dustin P: Microtubule poisons. in Microtubules (Book) Chapter 5. Pub Springer, N.Y.P., 1978, pp 167–184
Ehrlich HP, Ross R, Bornstein P: Effects of antimicrotubular agents on the secretion of collagen. J Cell Biol 62: 390–405, 1974
Rennard SI, Bitterman PB, Ozaki T, Rom WN, Crystal RG: Colchicine suppresses the release of fibroblast growth factors from alveolar macrophages in vitro. Am Rev Respir Dis 137: 181–185, 1988
Tanner MS, Jackson D, Mowat AP: Hepatic collagen synthesis in a rat model of cirrhosis, and its modification by colchicine. J Pathology 135: 179–187, 1981
Kirchenobich D, Uribe M, Suarez GI, Mata JM, Perez-Tamayo R, Rojkind M: Treatment of cirrhosis with colchicine: a double-blind randomized trial. Gastroenterology 77: 532–536, 1979
Kaplan MM, Alling DW, Zimmerman HJ, Wolfe HJ, Sepersky RA, Hirsch GS, Elta GH, Glick KA, Eagen KA: A prospective trial of colchicine for primary biliary cirrhosis. N Eng J Med 315: 1448–1454, 1986
Kershenobich D, Vargas F, Garcia-Tsao G, Tamayo RP, Gent M, Rojkind M: Colchicine in the treatment of cirrhosis of the liver. N Eng J Med 318: 1709–1713, 1988
Nath K, Shay JW, Bollon AP: Relationship between dibutryl cyclic AMP and microtubule organization in contracting heart muscle cells. Proc Natl Acad Sci USA 75: 319–323, 1978
Klein I, Levey JS, Gondel N: Characterization of the phosphoprotein profile in spontaneously beating cultured rat heart cells. Proc Soc Exptl Biol Med 170: 19–24, 1982
Klein I: Colchicine stimulates the rate of contraction of heart cells in culture. Cardiovasc Res 17: 459–465, 1983
Lampidis TJ, Trevorrow KW, Rubin RW: Effects of colchicine on cardiac cell function indicate possible role for membrane surface tubulin. Exptl Cell Res 164: 463–470, 1986
Lampidis TJ, Kolonias D, Savaraj N, Rubin RW: Cardiostimulatory and antiarrhythmic activity of tubulin-binding agents. Proc Natl Acad Sci USA 89: 1256–1260, 1992
Yonemochi H, Saikawa T, Takakura T, Ito, S, Takaki R: Effects of calcium antagonists on β-receptors of cultured cardiac myocytes isolated from neonatal rat ventricle. Circulation 81: 1401–1408, 1990
Limas C, Limas CL: Disparate effects of colchicine on thyroxine induced cardiac hypertrophy and adrenoceptor changes. Circ Res 68: 309–313, 1991
Tsutsui H, Ishihara K, Cooper IV G: Cytoskeletal role in contractile dysfunction of hypertrophied myocardium Science. 260: 682–687, 1993
Crie JS, Ord JM, Wakeland JR, Wildenthal K: Inhibition of cardiac proteolysis by colchicine. Biochem J 210: 63–71, 1983
Wildenthal K, Crie JS, Ord JM, Wakeland JR: The role of lysozymes and microtubules in cardiac protein degradation in Advances in Myocardiology 5: 137–144, 1985
Pfeffer JM, Pfeffer MA, Fishbein MC, Frohlich ED: Cardiac function and morphology with aging in the spontaneously hypertensive rat. Am J Physiol 237: H461-H468, 1979
Mirsky I, Pfeffer JM, Pfeffer MA, Braunwald E: The contractile state as the major determinant in the evolution of left ventricular dysfunction in the spontaneously hypertensive rat. Circ Res 53: 767–778, 1983
Conrad CH, Brooks VVW, Robinson KG, Bing OHL: Impaired myocardial function in spontaneously hypertensive rats with heart failure. Am J Physiol 260: H136-H145, 1991
Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OHL: Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circ 91: 161–170, 1995
Boluyt MO, O'Neill L, Meredith AL, Bing OHL, Brooks WW, Conrad CH, Crow MT, Lakatta EG: Alterations in gene cardiac expression during the transition from stable hypertrophy to heart failure. Circ Res 75: 23–32, 1994
Weber KT, Janicki JS, Shroff S, Pick R, Chen RM, Bashley RI: Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res 62: 757–765, 1987
Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP: Patterns of myocardial fibrosis. J Mol Cell Cardiol 21(Supp V): 121–131, 1989
Weber KT, Brilla CG: Structural remodeling of myocardial collagen in systemic hypertension: functional consequences and potential therapy. Heart Failure 6: 129–137, 1990
Weber KT, Brilla CG: Pathological hypertrophy and cardiac interstitium. Circulation 83: 1849–1865, 1991
Krebs HA, Hensclcit K: Untersuchungen uber die Harnstoffbildung im Tierkorper. Hoppe Seylers Z Physiol Chem. 210: 33–66, 1932
Yin FCP, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG: Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol 243: H941-H947, 1982
Sulman DL, Bing OHL, Mark RG, Burns SK: Physiologic loading of heart muscle. Biochem Biophys Res Comm 56: 947–951, 1974
Savitsky A, Golay MJE: Smoothing and differentiation of date by simplified least squares procedures. Analyt Chem 36: 1627–1639, 1964
Wiegner AW, Bing OHL: Laser scanner measurement of central segment performance in isolated cardiac muscle preparations. Am J Physiol 237: H260-H264, 1979
Mirsky I: Assessment of passive elastic stiffness of cardiac muscle: Mathematical concepts, physiologic and clinical considerations, directions of future research. Progr Card jovasc Dis 18: 277–308, 1976
Pasipoularides A, Mirsky I, Hess OM, Grimm J, Krayenbuehl HP: Myocardial relaxation and passive diastolic properties in man. Circulation 74: 991–1001, 1986
Bing OHL, Brooks WW, Conrad CH, Weinstein KB, Spadaro J, Radvany P: Myocardial mechanics of infarcted and hypertrophied noninfarcted myocardium following experimental coronary artery occlusion. In: R. Jacob, R.W. Gulch, G. Kissling (eds). Basic Research in Cardiology Cardiac Adaptation to Hemodynamic Overload. Training and Stress. Steinkopff Verlag, Darmstadt, 1983
Nakamura Y, Wiegner AW, Aslanis J, Apstein C, Bing OHL: Myocardial mechanics in allylamine induced myocardial fibrosis. Am J Physiol 251: H664-H669, 1986
Bergman L, Loxley R: Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Analyt Chem 35: 1961–1965, 1963
PROPHET Statistics: A User's Guide to Statistical Analysis on the PROPHET System. Cambridge, Massachusetts, BBN Systems and Technologies Corporation, 1988
Rochdi M, Sabouraud A, Baud FJ, Bismuth FJ, Scherrmann JM: Toxicokinetics of colchicine in humans: Analysis of tissue, plasma and urine data in ten cases. Human and Exp Toxicol 11: 510–516, 1992
Ertel N, Omokoku B, Wallace S: Colchicine concentration in leukocytes. ArthritisRheum 12: 293, 1969
Dayer JM, Roelke MS, Krane SM: Effects of prostaglandin E2, indomethacin, trifluoperazine and drugs affecting the cytoskeleton on collagenase production by cultured adherent rheumatoid synovial cells. Biochem Pharmacol 33: 2893–2899, 1984
Harris-ED Jr, Krane-SM: Collagenases (first of three parts). N Engl J Med Sept 291(11): 557–63, 1974
Berg RA, Schwartz ML, Crystal RG: Regulation of the production of secretory proteins: Intracellular degradation of newly synthesized ‘defective’ collagen. Proc Natl Acad Sci. USA 77: 4746–4750, 1980
The Merck Index. S. Budavari (ed.) Rahway, NJ, Merck & Co., Inc.; 3500–3501, 1989
Godeau G, Gonnord G, Wegrowski J, Pompidou A, Schovaert D, Robert L, Lagrue G, Robert AM: Effect of colchicine on atherosclerosis. III. Study of dermal elastic fibers by quantitative histochemistry, automated image analysis. Clin Physiol Biochem 3: 234–239, 1985
Jalil JE, Doering CW, Janicki JS, Pick R, Shroff SG, Weber KT: Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res 64: 1041–1050, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cicogna, A.C., Brooks, W.W., Hayes, J.A. et al. Effect of chronic colchicine administration on the myocardium of the aging spontaneously hypertensive rat. Mol Cell Biochem 166, 45–54 (1997). https://doi.org/10.1023/A:1006889126666
Issue Date:
DOI: https://doi.org/10.1023/A:1006889126666