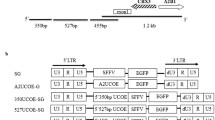
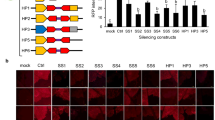
Abstract
Matrix attachment regions (MARs) are operationally defined as DNA elements that bind specifically to the nuclear matrix in vitro. It is possible, although unproven, that they also mediate binding of chromatin to the nuclear matrix in vivo and alter the topology of the genome in interphase nuclei. When MARs are positioned on either side of a transgene their presence usually results in higher and more stable expression in transgenic plants or cell lines, most likely by minimizing gene silencing. Our review explores current data and presents several plausible models to explain MAR effects on transgene expression.
Similar content being viewed by others
References
Able, J.A., Rathhus, C., Carroll, B.J. and Godwin, I.D. 2000. Enhancing transgene expression levels in sorghum: current status and future goals. In: N. Seetharama and I.D. Godwin (Eds.) Sorghum Tissue Culture, Transformation and Genetic Engineering, International Crops Research Institute for the Semi-Arid Tropics & Oxford Publishers (in press).
Al-Kaff, N.S., Covey, S.N., Kreike, M.M., Page, A.M., Pinder, R. and Dale, P.J. 1998. Transcriptional and posttranscriptional plant gene silencing in response to a pathogen. Science 279: 2113–2115.
Allen, G.C., Hall, G.E., Jr., Childs, L.C., Weissinger, A.K., Spiker, S. and Thompson, W.F. 1993. Scaffold attachment regions increase reporter gene expression in stably transformed plant cells. Plant Cell 5: 603–613.
Allen, G.C., Hall, G.E., Jr., Michalowski, S., Newman, W., Spiker, S., Weissinger, A.K. and Thompson, W.F. 1996. High-level transgene expression in plant cells: effects of a strong scaffold attachment region from tobacco. Plant Cell8: 899–913.
Anandalakshmi, R., Pruss, G.J., Ge, X., Marathe, R., Mallory, A.C., Smith, T.H. and Vance, V.B. 1998. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 95: 13079–13084.
Assaad, F.F., Tucker, K.L. and Signer, E.R. 1993. Epigenetic repeatinduced gene silencing (RIGS) in Arabidopsis. Plant Mol. Biol. 22: 1067–1085.
Avramova, Z., Sanmiguel, P., Georgieva, E. and Bennetzen, J.L. 1995. Matrix attachment regions and transcribed sequences within a long chromosomal continuum containing maize Adh1. Plant Cell 7: 1667–1680.
Avramova, Z., Tikhonov, A., Chen, M.S. and Bennetzen, J.L. 1998. Matrix attachment regions and structural colinearity in the genomes of two grass species. Nucl. Acids Res. 26: 761–767.
Beilmann, A., Albrecht, K., Shultze, S., Wanner, G. and Pfitzner, U.M. 1992. Activation of a truncated PR-1 promoter by endogenous enhancers in transgenic plants. Plant Mol. Biol. 18: 65–78.
Belmont, A.S. and Straight, A.F. 1998. In vivo visualization of chromosomes using lac operator-repressor binding. Trends Cell Biol. 8: 121–124.
Benham, C., Kohwi-Shigematsu, T. and Bode, J. 1997. Stressinduced duplex DNA destabilization in scaffold/matrix attachment regions. J. Mol. Biol. 274: 181–196.
Berezney, R. 1984. Organization and functions of the nuclear matrix. In: L.S. Hnilica (Ed.) Chromosomal Nonhistone Proteins, CRC Press, Boca Raton, FL, pp. 119–180.
Berezney, R. and Coffey, D.S. 1974. Identification of a nuclear protein matrix. Biochem. Biophys. Res. Comm. 60: 1410–1417.
Bidwell, J.P., Fey, E.G., Vanwijnen, A.J., Penman, S., Stein, J.L., Lian, J.B. and Stein, G.S. 1994. Nuclear matrix proteins distinguish normal diploid osteoblasts from osteosarcoma cells. Cancer Res. 54: 28–32.
Bode, J. and Maas, K. 1988. Chromatin domain surrounding the human interferon-β gene as defined by scaffold-attached regions. Biochemistry 27: 4706–4711.
Bode, J., Schlake, T., Rios-Ramirez, M., Mielke, C., Stengert, M., Kay, V. and Klehr-Wirth, D. 1995. Scaffold/matrix-attached regions: structural properties creating transcriptionally active loci. Int. Rev. Cytol. 162A: 389–454.
Bodnar, J.W. 1988. A domain model for eukaryotic DNA organization: a molecular basis for cell differentiation and chromosome evolution. J. Theor. Biol. 132: 479–507.
Bonifer, C., Hecht, A., Saueressig, H., Winter, D.M. and Sippel, A.E. 1991. Dynamic chromatin: the regulatory domain organization of eukaryotic gene loci. J. Cell. Biochem. 47: 99–108.
Bonifer, C., Vidal, M., Grosveld, F. and Sippel, A.E. 1990. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 9: 2843–2848.
Bonifer, C., Yannoutsos, N., Kruger, G., Grosveld, F. and Sippel, A.E. 1994. Dissection of the locus control function located on the chicken lysozyme gene domain in transgenic mice. Nucl. Acids Res. 22: 4202–4210.
Boulikas, T. 1995. Chromatin domains and prediction of MAR sequences. Int. Rev. Cytol. 162A: 279–388.
Breyne, P., Van Montague, M., Depicker, A. and Gheysen, G. 1992. Characterization of a plant scaffold attachment region in a DNA fragment that normalizes transgene expression in tobacco. Plant Cell 4: 463–471.
Brigneti, G., Voinnet, O., Li, W.X., Ji, L.H., Ding, S.W. and Baulcombe, D.C. 1998. Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J. 17: 6739–6746.
Bustin, M. and Reeves, R. 1996. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. In: W.E. Cohn and K. Moldave (Eds.) Progress in Nucleic Acid Research and Molecular Biology, Vol. 54, Academic Press, San Diego, CA, pp. 35–100.
Campisi, L., Yang, Y.Z., Yi, Y., Heilig, E., Herman, B., Cassista, A.J., Allen, D.W., Xiang, H.J. and Jack, T. 1999. Generation of enhancer trap lines in Arabidopsis and characterization of expression patterns in the inflorescence. Plant J. 17: 699–707.
Choi, J.Y., van Wijnen, A.J., Aslam, F., Leszyk, J.D., Stein, J.L., Stein, G.S., Lian, J.B. and Penman, S. 1998. Developmental association of the beta-galactoside-binding protein galectin-1 with the nuclear matrix of rat calvarial osteoblasts. J. Cell Sci. 111: 3035–3043.
Cockerill, P.N. and Garrard, W.T. 1986. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell 44: 273–282.
Conkling, M.A., Cheng, C.-L., Yamamoto, Y.T. and Goodman, H.M. 1990. Isolation of transcriptionally regulated root-specific genes from tobacco. Plant Physiol. 93: 1203–1211.
Cook, P.R. 1989. The nucleoskeleton and the topology of transcription. Eur. J. Biochem. 185: 487–501.
Cook, P.R. 1999. The organization of replication and transcription. Science 284: 1790–1795.
Cook, P.R. and Jackson, D.A. 1988. The nucleoskeleton: active site of transcription or artifact? In: K.W. Adolph (Ed.) Chromosomes and Chromatin, CRC Press, Boca Raton, FL, pp. 97–118.
Croft, J.A., Bridger, J.M., Boyle, S., Perry, P., Teague, P. and Bickmore, W.A. 1999. Differences in the localization and morphology of chromosomes in the human nucleus. J. Cell Biol. 145: 1119–1131.
Dorer, D.R. and Henikoff, S. 1994. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77: 993–1002.
Dutrillaux, B., Couturier, J., Richer, C.L. and Viegas-Pequignot, E. 1976. Sequence of DNA replication in 277 R-and Q-bands of human chromosomes using a BrdU treatment. Chromosoma 58: 51–61.
Elgin, S.C. 1988. The formation and function of DNase I hypersensitive sites in the process of gene activation. J. Biol. Chem. 263: 19259–19262.
Elmayan, T. and Vaucheret, H. 1996. Expression of single copies of a strongly expressed 35S transgene can be silenced posttranscriptionally. Plant J. 9: 787–797.
Fey, E.G. and Penman, S. 1988. Nuclear matrix proteins reflect cell type or origin in cultured human cells. Proc. Natl. Acad. Sci. USA 85: 121–125.
Gallie, D.R. 1998. Controlling gene expression in transgenics. Curr. Opin. Plant Biol. 1: 166–172.
Gasser, S.M. and Laemmli, U.K. 1986a. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of Drosophila melanogaster. Cell 46: 521–530.
Gasser, S.M. and Laemmli, U.K. 1986b. The organisation of chromatin loops: characterization of a scaffold attachment site. EMBO J. 5: 511–518.
Gasser, S.M. and Laemmli, U.K. 1987. A glimpse at chromosomal order. Trends Genet. 3: 16–22.
Gerdes, M.G., Carter, K.C., Moen, P.T. and Lawrence, J.B. 1994. Dynamic changes in the higher-level chromatin organization of specific sequences revealed by in situ hybridization to nuclear halos. J. Cell Biol. 126: 289–304.
Gindullis, F. and Meier, I. 1999. Matrix attachment region binding protein MFP1 is localized in discrete domains at the nuclear envelope. Plant Cell 11: 1117–1128.
Girard, F., Bello, B., Laemmli, U.K. and Gehring, W.J. 1998. In vivo analysis of scaffold-associated regions in Drosophila: a synthetic high-affinity SAR binding protein suppresses position effect variegation. EMBO J. 17: 2079–2085.
Gossen, M. and Bujard, H. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89: 5547–5551.
Greger, I.H. and Proudfoot, N.J. 1998. Poly(A) signals control both transcriptional termination and initiation between the tandem GAL10 and GAL7 genes of Saccharomyces cerevisiae. EMBO J. 17: 4771–4779.
Hall, G.E., Jr. and Spiker, S. 1994. Isolation and characterization of nuclear scaffolds. In: S. Gelvin and R.A. Schilperoort (Eds.) Plant Molecular Biology Manual, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 1–12.
Hall, G.E., Jr., Allen, G.C., Loer, D.S., Thompson, W.F. and Spiker, S. 1991. Nuclear scaffolds and scaffold-attachment regions in higher plants. Proc. Natl. Acad. Sci. USA 88: 9320–9324.
Han, K.H., Ma, C.P. and Strauss, S.H. 1997. Matrix attachment regions (MARs) enhance transformation frequency and transgene expression in poplar. Transgenic Res. 6: 415–420.
Hart, C.M. and Laemmli, U.K. 1998. Facilitation of chromatin dynamics by SARs. Curr. Opin. Genet. Dev. 8: 519–525.
Hassan, A.B., Errington, R.J., White, N.S., Jackson, D.A. and Cook, P.R. 1994. Replication and transcription sites are colocalized in human cells. J. Cell Sci. 107: 425–434.
Hatton, D. and Gray, J.C. 1999. Two MAR DNA-binding proteins of the pea nuclear matrix identify a new class of DNA-binding proteins. Plant J. 18: 417–429.
Huber, M. C., Bosch, F.X., Sippel, A.E. and Bonifer, C. 1994. Chromosomal position effects in chicken lysozyme gene transgenic mice are correlated with suppression of DNase I hypersensitive site formation. Nucl. Acids Res. 22: 4195–4201.
Ingelbrecht, I.L., Herman, L.M., Dekeyser, R.A., Van Montagu, M. and Depicker, A.G. 1989. Different 3 end regions strongly influence the level of gene expression in plant cells. Plant Cell 1: 671–680.
Ingelbrecht, I., Breyne, P., Vancompernolle, K., Jacobs, A., Van Montagu, M. and Depicker, A. 1991. Transcriptional interference in transgenic plants. Gene 109: 239–242.
Izaurralde, E., Käs, E. and Laemmli, U.K. 1989. Highly preferential nucleation of histone H1 assembly on sacffold-associated regions. J. Mol. Biol. 210: 573–585.
Jackson, D.A. 1997. Chromatin domains and nuclear compartments: establishing sites of gene expression in eukaryotic nuclei. Mol. Biol. Rep. 24: 209–220.
Jackson, D.A. and Cook, P.R. 1985. A general method for preparing chromatin containing intact DNA. EMBO J. 4: 913–918.
Jackson, D.A., Dickinson, P. and Cook, P.R. 1990a. Attachment of DNA to the nucleoskeleton of HeLa cells examined using physiological conditions. Nucl. Acids Res. 18: 4385–4393.
Jackson, D.A., Dickinson, P. and Cook, P.R. 1990b. The size of chromatin loops in HeLa cells. EMBO J. 9: 567–571.
John, S., Reeves, R.B., Lin, J.X., Child, R., Leiden, J.M., Thompson, C.B. and Leonard, W.J. 1995. Regulation of celltype-specific interleukin-2 receptor alpha-chain gene expression: potential role of physical interactions between Elf-1, HMG-I(Y), and NF-kappa B family proteins. Mol. Cell Biol. 15: 1786–1796.
Johnson, K.R., Disney, J.E., Wyatt, C.R. and Reeves, R. 1990. Expression of mRNAs encoding mammalian chromosomal proteins HMG-I and HMG-Y during cellular proliferation. Exp. Cell Res. 187: 69–76.
Jones, L., Hamilton, A.J., Voinnet, O., Thomas, C.L., Maule, A.J. and Baulcombe, D.C. 1999. RNA-DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 11: 2291–2301.
Kanda, T., Sullivan, K.F. and Wahl, G.M. 1998. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8: 377–385.
Käs, E., Izaurralde, E. and Laemmli, U.K. 1989. Specific inhibition of DNA binding to nuclear scaffolds and histone H1 by distamycin. The role of oligo(dA).oligo(dT) tracts. J. Mol. Biol. 210: 587–599.
Käs, E., Poljak, L., Adachi, Y. and Laemmli, U.K. 1993. A model for chromatin opening: stimulation of topoisomerase-II and restriction enzyme cleavage of chromatin by distamycin. EMBO J. 12: 115–126.
Kasschau, K.D. and Carrington, J.C. 1998. A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell 95: 461–470.
Kohwi-Shigematsu, T. and Kohwi, Y. 1990. Torsional stress stabilizes extended base unpairing in suppressor sites flanking immunoglobulin heavy chain enhancer. Biochemistry 29: 9551–9560.
Krauss, S.W., Larabell, C.A., Lockett, S., Gascard, P., Penman, S., Mohandas, N. and Chasis, J.A. 1997. Structural protein 4.1. in the nucleus of human cells: dynamic rearrangements during cell division. J. Cell Biol. 137: 275–289.
Levee, V., Garin, E., Klimaszewska, K. and Seguin, A. 1999. Stable genetic transformation of white pine (Pinus strobus L.) after cocultivation of embryogenic tissues with Agrobacterium tumefaciens. Mol. Breed. 5: 429–440.
Levy-Wilson, B. and Fortier, C. 1989. The limits of the DNase Isensitive domain of the human apolipoprotein B gene coincide with the location of chromosomal anchorage loops and define the 5' and 3' boundaries of the gene. J. Biol. Chem. 264: 21196–21204.
Liu, J.W. and Tabe, L.M. 1998. The influences of two plant nuclear matrix attachment regions (MARs) on gene expression in transgenic plants. Plant Cell Physiol. 39: 115–123.
Ma, H., Samarabandu, J., Devdhar, R.S., Acharya, R., Cheng, P.C., Meng, C.L. and Berezney, R. 1998. Spatial and temporal dynamics of DNA replication sites in mammalian cells. J. Cell Biol. 143: 1415–1425.
Marsden, M.P. and Laemmli, U.K. 1979. Metaphase chromosome structure: evidence for a radial loop model. Cell 17: 849–858.
Mette, M.F., van der Winden, J., Matzke, M.A. and Matzke, A.J.M. 1999. Production of aberrant promoter transcripts contributes to methylation and silencing of unlinked homologous promoters in trans. EMBO J. 18: 241–248.
Metzlaff, M., O'Dell, M., Cluster, P.D. and Flavell, R.B. 1997. RNA-mediated RNA degradation and chalcone synthase A silencing in Petunia. Cell 88: 845–854.
Michalowski, S.M., Allen, G.C., Hall, J.E., Jr., Thompson, W.F. and Spiker, S. 1999. Characterization of randomly-obtained matrix attachment regions (MARs) from higher plants. Biochemistry 38: 12795–12804.
Mielke, C., Kohwi, Y., Kohwi-Shigematsu, T. and Bode, J. 1990. Hierarchical binding of DNA fragments derived from scaffoldattached regions: correlation of properties in vitro and function in vivo. Biochemistry 29: 7475–7485.
Milot, E., Belmaaza, A., Wallenburg, J.C., Gusew, N., Bradley, W.E.C. and Chartrand, P. 1992. Chromosomal illegitimate recombination in mammalian cells is associated with intrinsically bent DNA elements. EMBO J. 11: 5063–5070.
Mirkovitch, J., Mirault, M.-E. and Laemmli, U. 1984. Organisation of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell 39: 223–232.
Mirkovitch, J., Spiere, P. and Laemmli, U.K. 1986. Genes and loops in 320,000 base-pairs of the Drosophila melanogaster chromosome. J. Mol. Biol. 190: 255–258.
Mirkovitch, J., Gasser, S.M. and Laemmli, U.K. 1987. Relation of chromosome structure and gene expression. Phil. Trans. R. Soc. Lond. Biol. 317: 563–574.
Misteli, T., Caceres, J.F. and Spector, D.L. 1997. The dynamics of a pre-mRNA splicing factor in living cells. Nature 387: 523–527.
Mlynárová, L., Loonen, A., Heldens, J., Jansen, R.C., Keizer, P., Stiekema, W.J. and Nap, J.P. 1994. Reduced position effect in mature transgenic plants conferred by the chicken lysozyme matrix-associated region. Plant Cell 6: 417–426.
Mlynárová, L., Jansen, R.C., Conner, A.J., Stiekema, W.J. and Nap, J.P. 1995. The MAR-mediated reduction in position effect can be uncoupled from copy number-dependent expression in transgenic plants. Plant Cell 7: 599–609.
Mlynárová, L., Keizer, L.C.P., Stiekema, W.J. and Nap, J.P. 1996. Approaching the lower limits of transgene variability. Plant Cell 8: 1589–1599.
Moreno Diaz de la Espina, S.M. 1995. Nuclear matrix isolated from plant cells. Int. Rev. Cytol. 162B: 75–139.
Odell, J.T. and Krebbers, E. 1998. Enhanced transgene expression in a population of monocot cells employing scaffold attachment regions. World Patent Office.
Olszewska, M.J. 1992. C-value paradox in angiosperm plant species. I. Sensitivity to DNase I in species with different 2C DNA content. Folia Histochem. Cytobiol. 30: 41–48.
Paul, A.L. and Ferl, R.J. 1998. Higher order chromatin structures in maize and Arabidopsis. Plant Cell 10: 1349–1359.
Paulson, J.R. and Laemmli, U.K. 1977. The structure of histonedepleted metaphase chromosomes. Cell 12: 817–828.
Phi-Van, L. and Strätling, W.H. 1996. Dissection of the ability of the chicken lysozyme gene 50 matrix attachment region to stimulate transgene expression and to dampen position effects. Biochemistry 35: 10735–10742.
Phi-Van, L., von Kries, J.P., Ostertag, W. and Strätling, W.H. 1990. The chicken lysozyme 5' matrix attachment region increases transcription from a heterologous promoter in heterologous cells and dampens position effects on the expression of transfected genes. Mol. Cell Biol. 10: 2302–2307.
Poljak, L., Seum, C., Mattioni, T. and Laemmli, U.K. 1994. SARs stimulate but do not confer position independent gene expression. Nucl. Acids Res. 22: 4386–4394.
Que, Q.D., Wang, H.Y., English, J.J. and Jorgensen, R.A. 1997. The frequency and degree of cosuppression by sense chalcone synthase transgenes are dependent on transgene promoter strength and are reduced by premature nonsense codons in the transgene coding sequence. Plant Cell 9: 1357–1368.
Rattray, A.J. and Symington, L.S. 1993. Stimulation of meiotic recombination in yeast by an ARS element. Genetics 134: 175–188.
Renz, M. 1975. Preferential and cooperative binding of histone I to chromosomal mammalian DNA. Proc. Natl. Acad. Sci. USA 72: 733–736.
Sabl, J.F. and Henikoff, S. 1996. Copy number and orientation determine the susceptibility of a gene to silencing by nearby heterochromatin in Drosophila. Genetics 142: 447–458.
Sawasaki, T., Takahashi, M., Goshima, N. and Morikawa, H. 1998. Structures of transgene loci in transgenic Arabidopsis plants obtained by particle bombardment: junction regions can bind to nuclear matrices. Gene 218: 27–35.
Schöffl, F., Schroder, G., Kliem, M. and Rieping, M. 1993. An SARsequence containing 395 bp DNA fragment mediates enhanced, gene-dosage-correlated expression of a chimaeric heat shock gene in transgenic tobacco plants. Transgenic Res 2: 93–100.
Spector, D.L. 1990. Higher order nuclear organization: threedimensional distribution of small nuclear ribonucleoprotein particles. Proc. Natl. Acad. Sci. USA 87: 147–151.
Sperry, A.O., Blasquez, V.C. and Garrard, W.T. 1989. Dysfunction of chromosomal loop attachment sites: illegitimate recombina-tion linked to matrix association regions and topoisomerase II. Proc. Natl. Acad. Sci. USA 86: 5497–5501.
Stalder, J., Larsen, A., Engel, J.D., Dolan, M., Groudine, M. and Weintraub, H. 1980. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNase I. Cell 20: 451–460.
Stam, M., Mol, J.N.M. and Kooter, J.M. 1997. The silence of genes in transgenic plants. Ann. Bot. 79: 3–12.
Stief, A., Winter, D.M., Strätling, W.H. and Sippel, A.E. 1989. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature 341: 343–345.
Straight, A.F., Marshall, W.F., Sedat, J.W. and Murray, A.W. 1997. Mitosis in living budding yeast: anaphase a but no metaphase plate. Science 277: 574–578.
Strick, R. and Laemmli, U.K. 1995. SARs are cis DNA elements of chromosome dynamics: synthesis of a SAR repressor protein. Cell 83: 1137–1148.
Sundaresan, V., Springer, P., Volpe, T., Haward, S., Jones, J.D.G., Dean, C., Ma, H. and Martienssen, R. 1995. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Gene Dev. 9: 1797–1810.
Takano, M., Egawa, H., Ikeda, J.E. and Wakasa, K. 1997. The structures of integration sites in transgenic rice. Plant J. 11: 353–361.
Thompson, A.J. and Myatt, S.C. 1997. Tetracycline-dependent activation of an upstream promoter reveals transcriptional interference between tandem genes within T-DNA in tomato. Plant Mol. Biol. 34: 687–692.
Thompson, E.M., Christians, E., Stinnakre, M.G. and Renard, J.P. 1994. Scaffold attachment regions stimulate HSP70.1 expression in mouse preimplantation embryos but not in differentiated tissues. Mol. Cell Biol. 14: 4694–4703.
Tikhonov, A.P., Bennetzen, J.L. and Avramova, Z.V. 2000. Structural domains and matrix attachment regions along colinear chromosomal segments of maize and sorghum. Plant Cell 12: 249–269.
Ñlker, B., Allen, G.C., Thompson, W.F., Spiker, S. and Weissinger, A.K. 1999. A tobacco matrix attachment region reduces the loss of transgene expression in the progeny of transgenic tobacco plants. Plant J. 18: 253–263.
Vain, P., Worland, B., Kohli, A., Snape, J.W., Christou, P., Allen, G.C. and Thompson, W.F. 1999. Matrix attachment regions increase transgene expression levels and stability in transgenic rice plants and their progeny. Plant J. 18: 233–242.
van der Geest, A.H.M., Hall, G.E., Jr. Spiker, S. and Hall, T.C. 1994. The beta-phaseolin gene is flanked by matrix attachment regions. Plant J. 6: 413–423.
van Drunen, C.M., Oosterling, R.W., Keultjes, G.M., Weisbeek, P.J., van Driel, R. and Smeekens, S.C.M. 1997. Analysis of the chromatin domain organisation around the plastocyanin gene reveals an MAR-specific sequence element in Arabidopsis thaliana. Nucl. Acids Res. 25: 3904–3911.
Van Holde, K. and Zlatanova, J. 1995. Chromatin higher order structure: chasing a mirage? J. Biol. Chem. 270: 8373–8376.
Vaucheret, H. 1993. Identification of a general silencer for 19S and 35S promoters in a transgenic tobacco plant: 90 bp of homology in the promoter sequence are sufficient for trans-inactivation. C. R. Acad. Sci. [III] 316: 1471–1483.
Vaucheret, H., Elmayan, T., Thierry, D., van der Geest, A., Hall, T., Conner, A.J., Mlynarova, L. and Nap, J.P. 1998. Flank matrix attachment regions (MARs) from chicken, bean, yeast or tobacco do not prevent homology-dependent trans-silencing in transgenic tobacco plants. Mol. Gen. Genet. 259: 388–392.
Verheijen, R., van Venrooij, W. and Ramaekers, F. 1988. The nuclear matrix: structure and composition. J. Cell Sci. 90: 11–36.
Verschure, P.J., van der Kraan, I., Manders, E.M.M. and van Driel, R. 1999. Spatial relationship between transcription sites and chromosome territories. J. Cell Biol. 147: 13–24.
Wan, K.M., Nickerson, J.A., Krockmalnic, G. and Penman, S. 1999. The nuclear matrix prepared by amine modification. Proc. Natl. Acad. Sci. USA 96: 933–938.
Weiler, K.S. and Wakimoto, B.T. 1995. Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29: 577–605.
Weintraub, H. and Groudine, M. 1976. Chromosomal subunits in active genes have an altered conformation. Science 193: 848–856.
Wolffe, A.P. 1997. Transcription control: Repressed repeats express themselves. Curr. Biol. 7: R796–R798.
Wolffe, A.P. and Matzke, M.A. 1999. Epigenetics: regulation through repression. Science 286: 481–486.
Ye, F. and Signer, E.R. 1996. RIGS (repeat-induced gene silencing) in Arabidopsis is transcriptional and alters chromatin configuration. Proc. Natl. Acad. Sci. USA 93: 10881–10886.
Zhao, K., Käs, E., Gonzalez, E. and Laemmli, U.K. 1993. SARdependent mobilization of histone H1 by HMG-I/Y in vitro: HMG-I/Y is enriched in H1–depleted chromatin. EMBO J. 12: 3237–3247.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Allen, G.C., Spiker, S. & Thompson, W.F. Use of matrix attachment regions (MARs) to minimize transgene silencing. Plant Mol Biol 43, 361–376 (2000). https://doi.org/10.1023/A:1006424621037
Issue Date:
DOI: https://doi.org/10.1023/A:1006424621037