Introduction
Both over- and undernutrition are associated with increased risk of chronic disease(Reference Soenen and Chapman1,Reference Michalakis, Goulis and Vazaiou2) and represent a major global public health issue. Appetite is the internal driving force for the ingestion of food(Reference de Graaf, Blom and Smeets3), and results from complex interactions between internal and external factors including biology, psychology and the environment. Along with environmental and psychological factors, knowledge of the biological processes involved in the control of food intake and appetite is therefore essential for better understanding and treatment of a range of conditions associated with poor appetite control, and hence over- and undernutrition (for example, poor appetite control associated with sedentary lifestyles, obesity or ageing).
Although several early theories attempted to explain appetite based on a single factor such as glucose, it has become increasingly evident that appetite is part of a larger integrative process and single-target approaches to understand or modify appetite control are largely ineffective. Appetite control has been conceptualised to consist of three levels of events and processes(Reference Blundell4) which interact to form part of a ‘psychobiological system’ controlling appetite(Reference Blundell and King5). These include (i) psychological events and behaviour, (ii) peripheral physiology and metabolic events, and (iii) neurotransmitter and metabolic interactions in the brain(Reference Blundell4).
Biomarkers of appetite are defined as physiological measures that relate to subjective appetite ratings, measured food intake, or both and can be considered indicators of appetite or causal factors influencing appetite(Reference de Graaf, Blom and Smeets3). Interest in identifying appetite biomarkers has continued to increase due to a number of potential applications. These include contributing to more objective and reliable measurement of appetite, increasing understanding of alterations in appetite across the lifespan and in health and disease, and identifying targets for improving appetite control. However, it should be noted that biomarkers are unable to fully characterise the range of processes involved in appetite control and should only be used to make claims about appetite in combination with behavioural measures(Reference Blundell, De Graaf and Hulshof6).
Several comprehensive reviews have previously discussed biomarkers of satiation and satiety in the brain and periphery(Reference de Graaf, Blom and Smeets3,Reference Havel7) and the role of the gastrointestinal tract and related peptide signals involved in the control of food intake(Reference Delzenne, Blundell and Brouns8). Satiation can be defined as the process leading to termination of eating, and satiety as the process leading to inhibition of further eating, a reduction in hunger and increase in fullness after a meal(Reference Blundell, De Graaf and Hulshof6). Examples of known biomarkers and potential biomarkers include gut hormones such as ghrelin and cholecystokinin (CCK), longer-term signals arising from adipose tissue such as leptin, along with gastric distension, cytokines such as IL-6 and the thermogenic effect of protein(Reference de Graaf, Blom and Smeets3,Reference Havel7,Reference Delzenne, Blundell and Brouns8) . Consensus statements note that biomarkers should be valid (clearly linked to appetite), reproducible, specific, sensitive and feasible – measured in accessible or easily obtained material using ethical and minimally invasive methods(Reference Diplock, Aggett and Ashwell9). This highlights the value that markers in blood or other easily obtained human samples such as saliva might have.
In addition to identification of gut hormones in blood, several circulating metabolites have long been implicated to have a key role in appetite control, with glucose being one of the first to be identified in Mayerʼs glucostatic theory of appetite in 1953(Reference Mayer10). Since then, many metabolites or small molecules present in biological samples have been proposed to be indirectly or causally associated with appetite, and consequently many studies have included metabolites as outcome measures (for example, Mennella et al. (Reference Mennella, Savarese and Ferracane11), Heini et al. (Reference Heini, Kirk and Lara-Castro12), Hall et al. (Reference Hall, Millward and Long13), Islam et al. (Reference Islam, Townsend and McKie14) and Karl et al. (Reference Karl, Smith and Wilson15)). However, most studies have targeted only one or a few specific metabolites. With the advancement of metabolomics in recent years our ability to measure a broad range of metabolites with diverse chemical characteristics has increased.
Given the complexity of appetite control, metabolomics offers great potential to increase understanding of the integrative processes of appetite control and to identify potential biomarkers of appetite. The aim of the present review is to (1) provide a collective overview of metabolites that have been identified in biological samples and associated with appetite control in humans and (2) discuss the potential for modern metabolomics techniques to identify appetite biomarkers and deepen understanding of appetite control.
The control of appetite and identified biomarkers
It is first important to acknowledge some of the key physiological processes involved in appetite control. For a seminal review, see de Graaf C et al. (Reference de Graaf, Blom and Smeets3). The hypothalamus plays a key role; in particular, the arcuate nucleus receives and processes signals both from other areas of the brain and the periphery. Briefly, the arcuate nucleus houses two sets of neuronal circuits that are functionally antagonistic. A group of neurons co-expressing neuropeptide Y (NPY) and agouti-related peptide (AgRP) are part of an appetite-stimulating (orexigenic) circuit. In contrast, pro-opiomelanocortin (POMC) and cocaine and amphetamine-regulated transcript (CART) neurons are part of an appetite-inhibiting (anorexigenic) circuit(Reference Wren and Bloom16) and signal to inhibit energy intake by action at specific melanocortin receptors(Reference Blundell17). There are also connections between the two neuronal sub-groups, for example, AgRP neurons can exert an inhibitory effect on POMC neurons(Reference Lau and Herzog18,Reference Atasoy, Betley and Su19) .
Signals from the periphery are often categorised as short term or long term but the connotation episodic and tonic is also appropriate(Reference Halford and Blundell20). Tonic signals such as leptin are constantly released, mainly by adipose tissue in proportion to the amount of lipid stores, therefore signalling chronic nutritional state(Reference Blundell17). Insulin, released by pancreatic β-cells, is also a tonic signal and shares many properties with leptin, with both stimulating POMC and inhibiting neuropeptide Y (NPY) to signal satiety. Leptin and insulin bind to their respective receptors on the surface of POMC neurons. This promotes processing of POMC to the mature hormone α-melanocyte-stimulating hormone (α-MSH), which binds to melanocortin-4 receptor and signals to decrease energy intake(Reference Baldini and Phelan21).
However, observations that leptin levels are elevated in many individuals with obesity have led to the hypothesis that most are resistant to the actions of leptin(Reference Caro, Sinha and Kolaczynski22), and similarly insulin resistance in individuals who are overweight and obese may mean a blunted effect of insulin on appetite(Reference Flint, Gregersen and Gluud23). It should also be noted that while tonic and episodic signals generally appear to have different roles in the control of appetite(Reference Blundell, Levin and King24) they can also interact with each other. For example, sensitivity to short-term signals can be influenced by leptin(Reference McMinn, Sindelar and Havel25), and may provide a mechanism through which long-term energy needs are translated into day-to-day food intake.
Episodic signals including orexigenic (ghrelin) and anorexigenic peptides (for example, CCK, glucagon-like peptide-1 (GLP-1) and peptide YY (PYY)), arise largely from the gastrointestinal tract and oscillate periodically in relation to eating(Reference Blundell, Levin and King24). Ghrelin circulates in acylated and deacylated forms; however, it is commonly measured as total ghrelin. Ghrelin stimulates appetite and rises before meals, suggesting a role in meal initiation(Reference Cummings, Purnell and Frayo26), whereas anorexigenic peptides are released in response to food ingestion. While there is some inconsistency in findings between studies(Reference Diepvens, Häberer and Westerterp-Plantenga27,Reference Smeets, Soenen and Luscombe-Marsh28) , relationships between gut peptides and appetite and energy intake at normal physiological levels have been demonstrated(Reference Lemmens, Martens and Kester29-Reference Adam and Westerterp-Plantenga32). For example, Gibbons et al. (Reference Gibbons, Caudwell and Finlayson30) found that plasma ghrelin (total and acylated) was positively correlated with changes in hunger and in turn food intake, and GLP-1 was negatively associated with hunger in the late satiety phase and subsequent food intake, following consumption of both high-fat and high-carbohydrate meals. These data suggest that ghrelin (total and acylated) and GLP-1 are significant biomarkers of the phases of satiety. In contrast, others(Reference Smeets, Soenen and Luscombe-Marsh28) have compared a high-protein (25 % energy) v. normal-protein (10 % energy) lunch, and found PYY, GLP-1 or acylated ghrelin did not explain the increased satiety response observed following the high-protein meal. Although not measured, other factors such as amino acids or other metabolites were proposed as factors which may explain the increased satiety response to the high-protein meal. These studies highlight the complexity of biological signals involved in meal-to-meal appetite control.
The large inter-individual variability in appetite and gut peptide responses to meal ingestion(Reference Gibbons, Hopkins and Beaulieu33) should also be acknowledged (see Fig. 1). One peptide biomarker is unlikely to fully explain appetite responses. It is more likely a combination of signals that influences appetite control, which could vary based on a range of factors such as the characteristics of the test meal or of the individual(Reference Gibbons, Hopkins and Beaulieu33). This highlights the importance of studying individual responses to a treatment or manipulation, as it may help to identify individuals or specific groups who might benefit, even though there may be no mean group changes(Reference Gibbons, Hopkins and Beaulieu33).
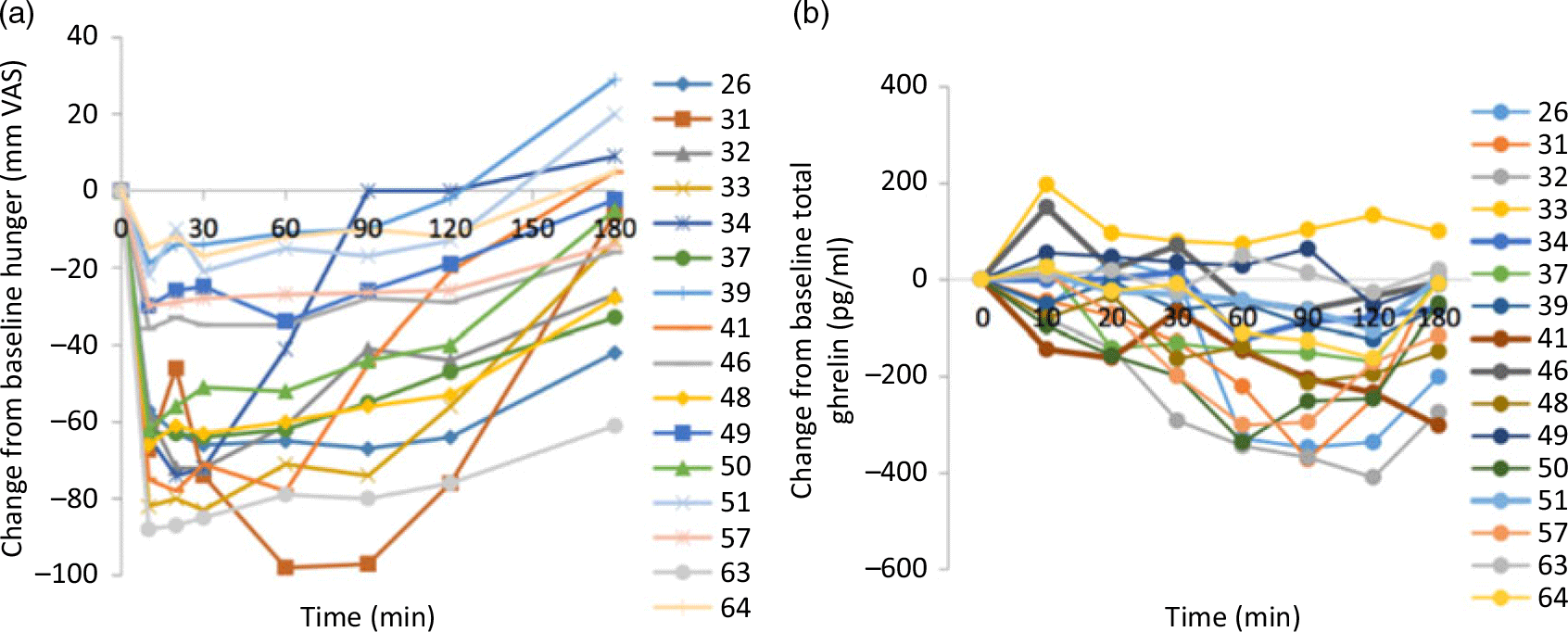
Fig. 1. Individual profiles for changes in (a) hunger and (b) total ghrelin in response to a high-fat test meal. From Gibbons et al. (Reference Gibbons, Hopkins and Beaulieu33). VAS, visual analogue scale.
Potential role of metabolomics
‘Omics’ methods including genomics, transcriptomics, proteomics and metabolomics have been applied in the search for biomarkers of a range of conditions. Metabolomics, including lipidomics specifically, involves the study of small molecules or metabolites present in biological samples(Reference Brennan34). Using multiple analytical techniques such as MS coupled with liquid chromatography or GC, and NMR spectroscopy, allows the characterisation of molecules such as carbohydrates, lipids, amino acids, bile acids and fatty acids. Each analytical technique has its own advantage and disadvantage and the optimal coverage of metabolites is obtained by use of multiple approaches. Metabolomics can be applied to a range of biological samples such as blood, urine, saliva, faecal water and cerebrospinal fluid.
Application of metabolomics in nutrition research has increased rapidly in recent years. In particular, it has played a key role in the following areas: (1) identification of biomarkers related to nutrient and food intake (food intake biomarkers); (2) understanding the impact of nutrition interventions to define potential mechanisms of action; (3) understanding diet–disease relationships in nutritional epidemiology; and (4) development of personalised nutrition (Fig. 2). Putative biomarkers exist for a range of foods including fish, red meat, citrus fruit, apples and cruciferous vegetables(Reference Gibbons, Michielsen and Rundle35-Reference Andersen, Kristensen and Manach40). The goal of food intake biomarkers is to aid in the assessment of food intake as self-reported methods have well-accepted limitations(Reference Bingham41,Reference Kipnis, Midthune and Freedman42) . Work using proline betaine as a biomarker of citrus intake demonstrated that using these biomarkers one can obtain quantitative information on food intake(Reference Gibbons, Michielsen and Rundle35). In this study participants consumed standardised breakfasts as part of a controlled dietary intervention for 3 consecutive days over 3 weeks where citrus intake was changed over the weeks. The urinary proline betaine concentrations were used to develop calibration curves relating citrus intake with biomarker levels. These curves were then used to determine citrus intake in an independent cross-sectional study of 560 individuals and the results demonstrated that the biomarker approach performed extremely well at determining intake. In a similar study setting, Garcia-Perez et al. (Reference Garcia-Perez, Posma and Chambers43) examined the ability of tartaric acid to determine grape intake. While development of these and other biomarkers is important it is also worth noting that they will not completely replace self-reported data and the true value will be in combining both approaches.
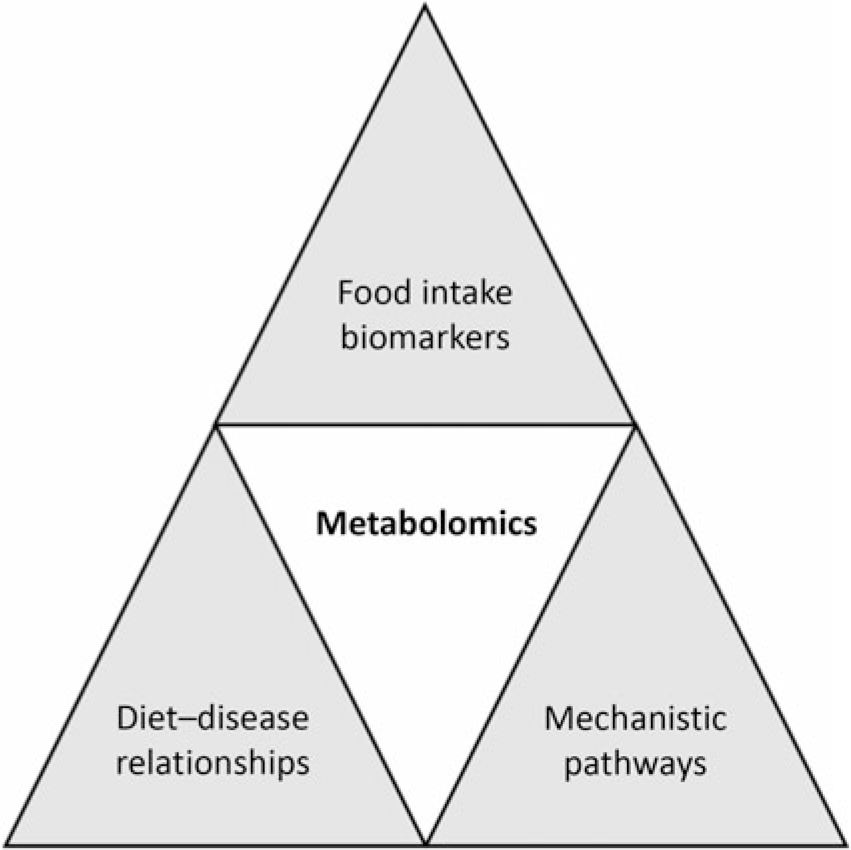
Fig. 2. Potential applications of metabolomics in nutrition research. Metabolomics has been used to identify biomarkers of food intake; examples now exist for a range of foods including but not limited to fish, red meat, citrus fruit, apples and cruciferous vegetables. Diet–disease relationships can be examined through application of metabolomics. In addition, through the identification of metabolic pathways altered following nutrition interventions, mechanistic insights can be obtained.
Metabolites associated with appetite control
Although several metabolites including lipids and amino acids have been shown to be associated with subjective appetite ratings or measured food intake in human studies (Table 1), most studies that have investigated potential biomarkers of appetite have been hypothesis driven and focused on measuring a single metabolite or a limited number of specific metabolites.
Table 1. Metabolites that have been identified in human biofluids and associated with subjective appetite ratings and/or energy intake (EI)*
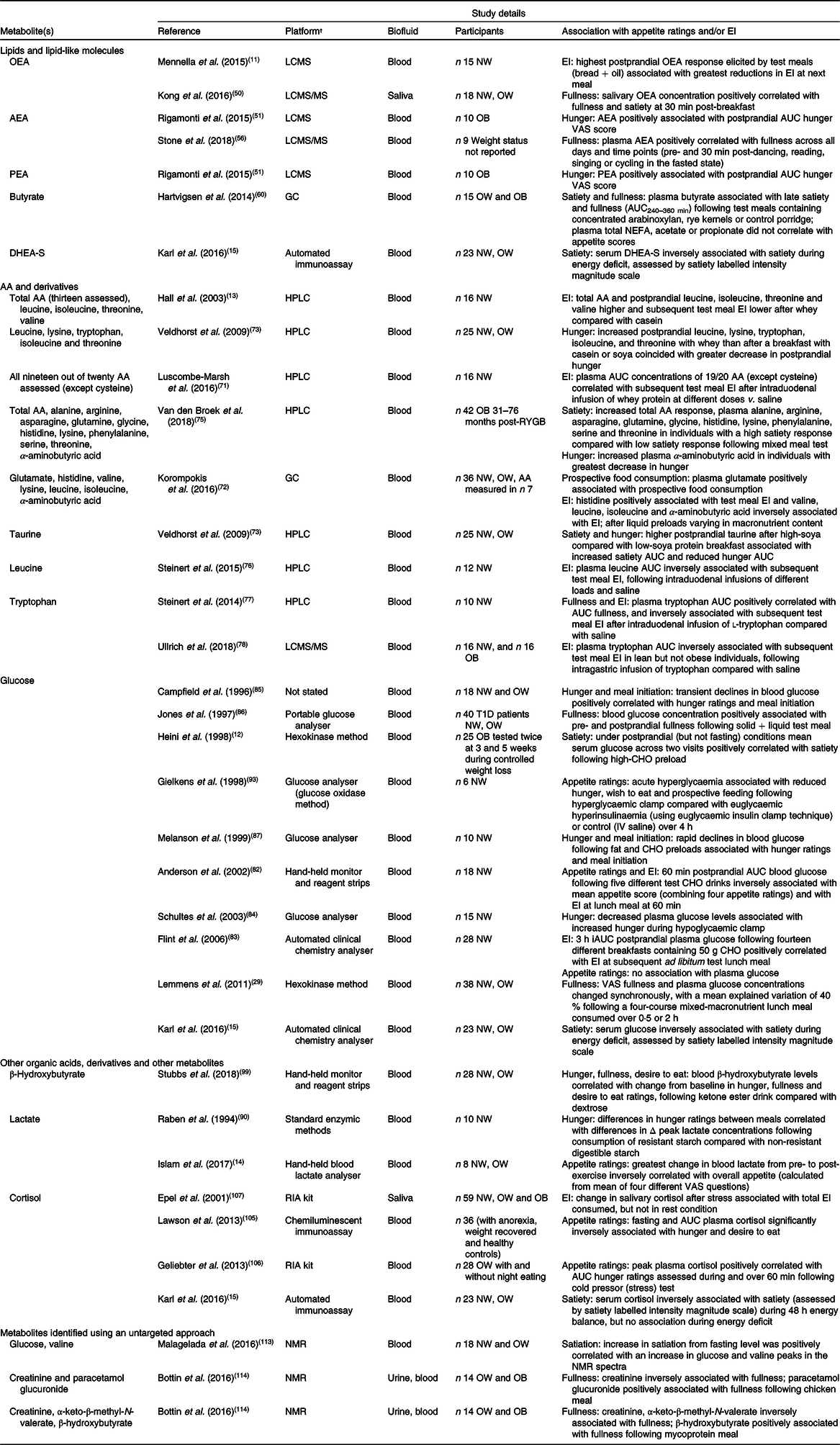
OEA, oleoylethanolamide; LCMS, liquid chromatography MS; NW, normal weight; OW, overweight; AEA, anandamide; OB, obese; VAS, visual analogue scale; PEA, palmitoylethanolamide; DHEA-S, dehydroepiandrosterone-sulfate; AA, amino acid; RYGB, Roux-en-Y gastric bypass surgery; T1D, type 1 diabetes; CHO, carbohydrate; IV, intravenous; iAUC, incremental AUC.
* Metabolites were identified using targeted searches of the Human Metabolome Database, research databases including OVID Medline and Google Scholar and reference lists of key articles. Combinations of the following key terms were included: metabolites; metabolomics; glucose; amino acids; fatty acids; bile acids; carbohydrates; appetite; hunger; fullness; satiation; satiety; energy intake; food intake. Articles that reported direct associations between metabolites and appetite and/or energy intake in humans were included.
† Platform/method of analysis as described in original paper.
Lipids, lipid-like molecules and fatty acids
Circulating concentrations of total levels of NEFA have long been associated with appetite control. In 1960, van Itallie & Hashim(Reference Van Itallie and Hashim44) showed that patterns of plasma NEFA in individuals on self-selected diets were similar to patterns of hunger and satiety. More recently, plasma NEFA concentrations have been shown to predict differences in the duration of satiety following ingestion of a pharmacological agent known to inhibit NEFA β-oxidation compared with an agent known to stimulate NEFA β-oxidation(Reference Gatta, Zuberbuehler and Arnold45). In contrast, others have shown plasma NEFA levels to change without changes in appetite in response to overfeeding in humans(Reference Deighton, King and Matu46), illustrating that NEFA levels may only be associated with appetite under certain conditions. Regarding individual NEFA, there is substantial mechanistic evidence for a role in appetite control from animal studies. For example, central administration of the long-chain fatty acid oleic acid has been shown to reduce food intake in rats(Reference Obici, Feng and Morgan47). However, excessive intake of long-chain SFA can promote hypothalamic insulin and leptin resistance(48) which could in turn blunt effects on appetite. In humans, ingestion of specific fatty acids has been shown to have an impact on appetite(Reference Lawton, Delargy and Brockman49). However, whether circulating concentrations of individual NEFA in humans are associated with appetite or food intake remains to be clearly established.
Interestingly, fatty acid ethanolamides, a class of lipid-signalling molecules derived from fatty acid precursors, such as oleoylethanolamide (OEA, the ethanolamide of oleic acid)(Reference Mennella, Savarese and Ferracane11,Reference Kong, Ferracane and De Luca50) , anandamide (AEA) of arachidonic acid(Reference Rigamonti, Piscitelli and Aveta51) and palmitoylethanolamide of palmitic acid(Reference Rigamonti, Piscitelli and Aveta51) have been shown to be associated with appetite in humans (Table 1). OEA is an N-acylethanolcholamine, with most of its reported effects being attributed to activation of PPAR-α(Reference Fu, Gaetani and Oveisi52) in the small intestine(Reference Fu, Kim and Oveisi53). Although the mechanisms linking PPARα activation to satiety are incompletely understood, the subsequent activation of apoA-IV(Reference Tso and Liu54), stimulation of CCK release and inhibition of gastric motility(Reference Glatzle, Darcel and Rechs55) may have a role.
In a human study, Mennella et al. (Reference Mennella, Savarese and Ferracane11) examined the effects of fatty acid composition of a meal (white bread with three different test oils), particularly its oleic acid content, on plasma OEA along with four other related lipid molecules, on appetite and energy intake. Meals eliciting the highest plasma OEA response resulted in reduced intake at a lunch meal served 3 h later. Others, such as Kong et al. (Reference Kong, Ferracane and De Luca50), have since shown salivary OEA concentrations to be positively associated with satiety and fullness. Collectively, although human studies are limited, and others have not reported such associations(Reference Rigamonti, Piscitelli and Aveta51), these studies combined with mechanistic evidence from animal studies highlight OEA in blood and saliva as a potential biomarker of appetite following consumption of certain foods in humans.
AEA, an endogenous agonist of the cannabinoid CB1 and CB2 receptors, and the AEA metabolically related lipid palmitoylethanolamide (PEA) and agonist of PPARα have also been shown to positively correlate with postprandial hunger ratings(Reference Rigamonti, Piscitelli and Aveta51), although not in all studies(Reference Kong, Ferracane and De Luca50). The postprandial AUC of plasma AEA and PEA were shown to positively correlate with postprandial hunger AUC in ten men with obesity in an already satiated state(Reference Rigamonti, Piscitelli and Aveta51). Plasma AEA was also shown to positively correlate with ratings of fullness in the fasted state in healthy postmenopausal women(Reference Stone, Millar and Herrod56). Overall, while there is substantial evidence for the role of these endocannabinoids and related lipid molecules in the control of appetite and food intake in animal studies, studies in humans examining the role of circulating levels of these metabolites are currently limited.
SCFA, including acetate, butyrate and propionate, are produced when non-digestible carbohydrate is fermented in the colon and appear to have a beneficial role in appetite control and energy homeostasis (for a detailed review, see Byrne et al. (Reference Byrne, Chambers and Morrison57)). Various factors must be considered when interpreting their circulating SCFA concentrations including that they can be produced from both endogenous and exogenous sources. However, in general colonic fermentation is considered the main source of acetate in the blood(Reference Wolever, Josse and Leiter58). A direct role of acetate in central appetite control has been shown in mice, with findings that peripheral administration of acetate increased POMC and reduced AgRP expression in the hypothalamus(Reference Frost, Sleeth and Sahuri-Arisoylu59).
Few studies have directly investigated relationships between concentrations in human biofluids of individual SCFA and appetite or energy intake. In one study, plasma butyrate has been shown to correlate directly with late satiety and fullness 4–6 h after a range of test breakfasts(Reference Hartvigsen, Lærke and Overgaard60). Others have shown targeted delivery of propionate to the colon through ingestion of an inulin-propionate ester increased plasma propionate, GLP-1 and PYY levels, and reduced subsequent energy intake(Reference Chambers, Viardot and Psichas61), but direct correlations between plasma propionate and energy intake were not reported. Elsewhere(Reference Byrne, Preston and Brignardello62), the non-digestible carbohydrate l-rhamnose was found to significantly increase plasma propionate and PYY, but had no effect on appetite. The latter finding may be due to the small sample size (n 10). As the composition of the gut microbiota can shape the faecal metabolome(Reference Ilhan, DiBaise and Isern63), the faecal metabolome also presents a useful approach for further understanding of the role of fatty acids as biomarkers in appetite control. There is evidence of higher faecal concentrations of proprionate, butyrate and branched-chain fatty acids (isobutyrate and isovalerate) in individuals with obesity post-gastric bypass(Reference Ilhan, DiBaise and Isern63) suggesting a potential mechanism by which gastric bypass surgery may have an impact on satiety. However, appetite was not assessed.
Dehydroepiandrosterone sulfate (DHEA-S), produced from cholesterol belonging to the class of compounds known as sulfated steroids (sterol lipids with a sulfate group), has been shown to be inversely correlated with satiety ratings during energy deficit over 48 h in soldiers(Reference Karl, Smith and Wilson15). DHEA-S modulates the activity of neurotransmitters within regions in the central nervous system involved in appetite control(Reference Maninger, Wolkowitz and Reus64); however, as it does not readily cross the blood–brain barrier, changes in peripheral concentrations may not alter concentrations in the brain(Reference Karl, Smith and Wilson15). Therefore, while it may potentially serve as an indirect marker of satiety under certain conditions, the role of circulating levels in appetite control in humans is currently unclear and needs further investigation.
Taken together, there is emerging evidence for a role of some lipid molecules including fatty acid ethanolamides as potential biomarkers of appetite in humans. Moreover, while there appears to be a clear role for individual fatty acids such as oleic acid and SCFA in appetite control, with substantial mechanistic evidence in animals, evidence of direct associations between concentrations of individual fatty acids in biofluids such as blood or faeces and appetite/energy intake in humans is currently lacking.
Amino acids
Circulating concentrations of amino acids have long been implicated to have a role in appetite control. In 1956, Mellinkoff(Reference Mellinkoff, Frankland and Boyle65) demonstrated that when serum amino acid nitrogen increased, appetite diminished and when amino acid concentration decreased, appetite increased following a breakfast of eggs, milk and toast. Various mechanisms have since been proposed linking amino acids to appetite control, including the original amino-static hypothesis(Reference Mellinkoff, Frankland and Boyle65) – that amino acid levels in general are monitored by some kind of amino-stat, and that when a certain level is reached this limits the intake of further amino acids (hence food)(Reference Hill, Blundell and Huether66). Millwardʼs(Reference Millward67) ‘protein-stat’ theory further suggests that skeletal muscle mass specifically is tightly regulated and food intake is directed to meet the needs for lean tissue growth and maintenance. However, the evidence for such regulation is currently limited, mainly due to a lack of research(Reference Hopkins, Blundell and Harris68).
Although findings are inconsistent, some studies have since reported associations between single circulating amino acids or multiple amino acids with subjective appetite ratings and energy intake in humans (Table 1). For example, Hall et al. (Reference Hall, Millward and Long13) reported that a greater rise in total amino acids, CCK and GLP-1 occurred following whey compared with casein consumption and energy intake at an ad libitum meal 90 min later was concomitantly reduced. When separately examined, valine, isoleucine, leucine and threonine were the amino acids that significantly differed. The authors suggested that the influence of the amino acid and gut peptide response on satiety was probably complementary. In contrast, in men with overweight and obesity during energy restriction, no strong association was evident between circulating plasma amino acid concentrations and appetite(Reference Lobley, Holtrop and Horgan69,Reference Neacsu, Fyfe and Horgan70) . This indicates no direct action of circulating amino acids on central mechanisms of satiety under these conditions(Reference Lobley, Holtrop and Horgan69,Reference Neacsu, Fyfe and Horgan70) , leading to the suggestion that complex and redundant pathways may be involved in protein- and amino acid-induced satiety(Reference Neacsu, Fyfe and Horgan70).
Others have shown that postprandial plasma concentrations of all twenty amino acids, except cysteine, were positively associated with suppression of subsequent energy intake in healthy lean males following intra-duodenal infusion of whey protein(Reference Luscombe-Marsh, Hutchison and Soenen71), which may argue against independent roles for individual amino acids in appetite control. However, elsewhere, specific plasma amino acid concentrations were demonstrated to correlate with prospective food consumption (glutamate) and energy intake (EI) (histidine, valine, leucine, isoleucine and the amino acid derivative α-aminobutyric acid) following differing liquid preloads with varied macronutrient composition in normal-weight and overweight adults(Reference Korompokis, Östman and Dougkas72). Others have shown that taurine was the only amino acid to directly correlate with increased satiety and reduced hunger following consumption of soya protein in humans(Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen73). Such an effect could also potentially explain the satiating effects of fish that have been previously observed, as seafood is rich in taurine(Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen73,Reference Uhe, Collier and O’Dea74) . These studies highlight a potential role for different amino acids in appetite control following consumption of different foods.
Amino acids and amino acid derivatives have also been recently implicated in the increased satiety response following Roux-en-Y gastric bypass (RYGB) surgery(Reference Van den Broek, de Heide and Emous75). In this cross-sectional study, participants were divided into those with high and low postprandial satiety scores and high and low postprandial reductions in hunger. Those with high satiety scores had a significantly greater total amino acid response and greater response of ten out of twenty-four individual amino acids, along with greater GLP-1 and PYY responses. Five of these amino acids were particularly pronounced in those with high GLP-1 and PYY responses, suggesting this may have been the mechanism of action for those amino acids. However, it was proposed that the other five amino acids that differed may have a direct action on satiety. Interestingly, when divided into groups based on high and low reductions in hunger, only one metabolite differed – the amino acid derivative α-aminobutyric acid, highlighting that amino acids may have varying associations with different appetite sensations (i.e. hunger v. satiety). However, as responses were assessed at one time point only post-operatively, causal relationships between changes in circulating amino acid concentrations and appetite could not be established.
Finally, some studies have investigated the role of specific amino acids in appetite control through intraduodenal infusions of certain amino acids and subsequent measurement of plasma concentrations. Steinert et al. (Reference Steinert, Landrock and Ullrich76,Reference Steinert, Luscombe-Marsh and Little77) reported circulating levels of plasma leucine and tryptophan to be inversely associated with subsequent test meal energy intake following intraduodenal infusions of leucine(Reference Steinert, Landrock and Ullrich76) and tryptophan(Reference Steinert, Luscombe-Marsh and Little77), respectively, compared with saline. Elsewhere it has been shown that this may be population specific, with associations between plasma tryptophan and test meal energy intake following intra-duodenal tryptophan infusion being only apparent in lean individuals but not in those with obesity(Reference Ullrich, Fitzgerald and Giesbertz78).
Collectively, these human studies highlight that circulating amino acids could have a role in appetite control and as a potential biomarker of appetite in certain contexts, and that this may occur either indirectly through effects of gut peptides or directly, or a combination of both.
Glucose
The glucostatic hypothesis, proposing a role for blood glucose levels and glucose utilisation in the regulation of appetite, was also described in the 1950s(Reference Mayer10,Reference Mayer79) , and has since been the focus of substantial research. An extensive review of the role of glucose in appetite control is beyond the scope of the present review (for reviews, see Campfield & Smith(Reference Campfield and Smith80) and Chaput & Tremblay(Reference Chaput and Tremblay81)). Various studies in humans have demonstrated that postprandial blood glucose concentrations or patterns of blood glucose are associated with appetite ratings, energy intake at a test meal and/or meal initiation (see Table 1 (Reference Heini, Kirk and Lara-Castro12,Reference Karl, Smith and Wilson15,Reference Flint, Gregersen and Gluud23,Reference Lemmens, Martens and Kester29,Reference Anderson, Catherine and Woodend82-Reference Melanson, Westerterp-Plantenga and Saris87) ). However, it is essential to note that many studies have also shown no association between blood glucose concentrations and appetite ratings(Reference Flint, Gregersen and Gluud23,Reference Flint, Møller and Raben83,Reference Schultes, Panknin and Hallschmid88-Reference Raben, Tagliabue and Christensen90) .
The inconsistent findings may be due to several reasons. Like many postprandial markers, distinguishing between the contributions of individual signals is difficult. For example, during controlled weight loss in women with obesity, when examined in multivariate regression analyses, insulin was the greatest and only independent predictor of satiety(Reference Heini, Kirk and Lara-Castro12), supporting an insulinotropic(Reference Porte and Woods91) rather than glucostatic hypothesis. Further evidence suggesting that the role of glucose in appetite control may occur indirectly through effects on other mechanisms comes from studies that have shown intraduodenal glucose infusion to suppress appetite and reduce EI(Reference Lavin, Wittert and Sun92) but intravenous glucose infusion to have no effect(Reference Schultes, Panknin and Hallschmid88,Reference Lavin, Wittert and Sun92) . These findings together with evidence from a meta-analysis(Reference Flint, Gregersen and Gluud23) suggest that the impact of intestinal glucose is unlikely to be mediated by effects on blood glucose, but instead via effects on insulin and/or incretins(Reference Flint, Gregersen and Gluud23,Reference Schultes, Panknin and Hallschmid88) .
A further explanation is that blood glucose concentrations may only have an impact on appetite and energy intake at extreme levels such as during hypoglycaemia or in hyperglycaemia, but not when blood glucose levels are within the normal physiological range. Indeed, several studies reporting associations between appetite, energy intake and glucose showed that such associations existed in individuals with type 1 diabetes(Reference Jones, Horowitz and Berry86), during a hypoglycaemic clamp(Reference Schultes, Oltmanns and Kern84), or during acute hyperglycaemia(Reference Gielkens, Verkijk and Lam93). For example, some hypoglycaemic clamp studies have shown that hunger ratings are increased at blood glucose levels of 3·0 mmol/l(Reference Schultes, Oltmanns and Kern84,Reference Schultes, Kern and Oltmanns94) .
In summary, it is clear that blood glucose may be associated with appetite under certain conditions, such as at extreme levels during hypoglycaemia or hyperglycaemia. However, at normal physiological levels, findings are generally inconsistent and interpreting the association of glucose with appetite is often confounded by covariation with other key postprandial markers involved in appetite control.
Other organic acids, derivatives and other metabolites
In addition to fatty acids, amino acids and glucose, several other metabolites such as ketone bodies, lactate and cortisol have been associated with appetite. Alterations in appetite with ketosis have been thought to have a major role in contributing to weight loss. During ketosis, there is an increase in circulating concentrations of ketones (β-hydroxybutyrate, acetoacetate and acetone) that are synthesised as a consequence of a sustained increase in β-oxidation of NEFA in the liver, and provide an alternative fuel source when glucose supply is limited, for example, during prolonged periods of fasting or very low-carbohydrate diet(Reference Gibson, Seimon and Lee95). In a meta-analysis examining changes in appetite during ketosis, hunger and desire to eat were shown to be suppressed and fullness/satiety increased, with ketosis appearing to provide a plausible explanation for this(Reference Gibson, Seimon and Lee95). However, whether there is a threshold level of circulating ketone concentrations at which appetite is suppressed could not be determined as the level of β-hydroxybutyrate (about 0·5 mm) was similar among all studies included(Reference Gibson, Seimon and Lee95). Nevertheless, there is mechanistic evidence from animal studies that intracerebroventricular infusion(Reference Arase, Fisler and Shargill96) and subcutaneous injection(Reference Langhans, Egli and Scharrer97) of β-hydroxybutyrate reduce food intake, along with support from in vitro studies showing that β-hydroxybutyrate under physiological conditions influences AgRP expression and reduces orexigenic signalling via the AMPK pathway(Reference Laeger, Pöhland and Metges98).
Promising data in humans for a direct effect of circulating ketones on appetite come from evidence of reductions in appetite following consumption of a taste-matched ketone ester drink compared with a dextrose drink(Reference Stubbs, Cox and Evans99). In response to consumption of the ketone ester drink, β-hydroxybutyrate levels increased from 0·2 to 3·3 mm after 1 h, the onset of hunger was delayed, desire to eat reduced and there was a delayed rise in plasma total ghrelin levels. Moreover, β-hydroxybutyrate circulating concentrations were strongly inversely correlated with change from baseline in hunger and desire to eat, and positively correlated with fullness. Interestingly, the appetite-suppression effect in this study could not be attributed to plasma glucose, insulin, GLP-1 or PYY levels. Overall, although human studies examining direct effects are currently limited, the evidence to date indicates blood β-hydroxybutyrate as a promising potential biomarker for appetite, which may act either directly or indirectly through effects on gut hormones such as ghrelin.
Lactate, produced from pyruvate, has a role in many biological processes including as an energy substrate, and is another metabolite that appears to have a role in appetite control. Plasma lactate concentrations have been shown to increase after meals in a similar pattern to insulin, with a greater response following consumption of high- compared with low-carbohydrate meals(Reference Havel, Townsend and Chaump100). Although appetite ratings were not assessed in that study, others have shown direct correlations between circulating lactate concentrations and hunger ratings following consumption of resistant starch(Reference Raben, Tagliabue and Christensen90). In a subsequent analysis by the same authors(Reference Raben, Holst and Christensen101) using data from three studies combined, Δ mean satiety was most strongly correlated with Δ AUC plasma lactate, and was also correlated with glucose, insulin, noradrenaline, gastric inhibitory peptide and carbohydrate oxidation. In a more recent study, blood lactate was implicated to have a role in ‘exercise-induced anorexia’ – the transient suppression of appetite following high-intensity exercise, with an increase in blood lactate inversely correlated with both subjective appetite ratings and acylated ghrelin AUC(Reference Islam, Townsend and McKie14).
As lactate binds to the G protein-coupled receptor on gastric cells that produce ghrelin, and inhibits ghrelin secretion(Reference Engelstoft, Park and Sakata102), this may be one mechanism by which lactate has an impact on appetite control. In addition, findings from animal studies indicate that lactate may act centrally in the regulation of food intake(Reference Lam, Chari and Wang103). The influence of lactate on appetite, however, may also be dependent on circulating levels of other metabolites such as glucose, with evidence of reductions in energy intake when lactate was infused during a euglycaemic clamp but no effects during a hypoglycaemic clamp in humans(Reference Schultes, Schmid and Wilms104). Overall, the current evidence in humans, supported by mechanistic studies in both humans and animals, indicates that lactate may have a role in appetite control under certain conditions.
Glucocorticoids such as cortisol, a steroid hormone and the major glucocorticoid secreted by the adrenal gland, has been shown to directly correlate with appetite ratings(Reference Karl, Smith and Wilson15,Reference Lawson, Holsen and Desanti105,Reference Geliebter, Carnell and Gluck106) and energy intake(Reference Epel, Lapidus and McEwen107) in some, but not all(Reference Raspopow, Abizaid and Matheson108) studies. Serum cortisol was inversely correlated with satiety during a period of 48 h of energy balance in humans; however, there was no relationship during an equivalent period of energy deficit(Reference Karl, Smith and Wilson15). In addition, salivary and plasma cortisol were shown to be associated with increased hunger(Reference Geliebter, Carnell and Gluck106) and energy intake(Reference Epel, Lapidus and McEwen107) in women under conditions of stress, but not on a rest day (without stress)(Reference Epel, Lapidus and McEwen107). Cortisol responses to food intake have also been correlated with acylated ghrelin in women with obesity, suggesting a common neuro-humoral pathway through which stress and anxiety may influence appetite(Reference Sarker, Franks and Caffrey109). However, it may be likely that cortisol reflects or modulates other factors that respond to stress, such as leptin, neuropeptide Y (NPY), or cytokines, that have a more direct effect on appetite, than directly influencing appetite itself(Reference Epel, Lapidus and McEwen107).
Bile acids
Bile consists of a range of molecules including bile acids, cholesterol, phospholipids and bilirubin. In addition to key roles in lipid metabolism and cholesterol homeostasis, bile acids can act as signalling molecules and an effect of bile acids on appetite was described in 1968(Reference Bray and Gallagher110). More recent studies have shown bile acids in plasma to correlate with key appetite-related gut hormones. For example, following a standardised test meal in twelve normal-weight adults GLP-1 and PYY responses were found to correlate with chenodeoxycholic acid metabolites while total ghrelin was inversely correlated with deoxycholic acid metabolites(Reference Roberts, Glicksman and Alaghband-Zadeh111). However, appetite was not assessed. Therefore, while there is evidence for a role of bile acids in appetite control, and this may occur through direct effects or indirectly through effects on gut hormones (for a detailed discussion, see Kuhre et al. (Reference Kuhre, Albrechtsen and Larsen112)), current findings showing direct associations between bile acid concentrations in biological samples and appetite/energy intake in humans are limited.
Untargeted metabolomics approaches and appetite
In contrast to studies targeting specific metabolites, current metabolomics techniques allow for hundreds of metabolites to be quantified and also allow an untargeted approach to be employed where one has the potential to identify new/novel biomarkers. There are limited studies that have employed metabolomics-based approaches to the study of appetite. Using NMR for metabolomics analysis, Malagelada et al. (Reference Malagelada, Barba and Accarino113) found increased plasma valine and glucose levels to correlate with satiation immediately after ingestion of a test meal to satiation. In addition, circulating metabolites were proposed that could serve as objective biomarkers of hedonic responses to food ingestion(Reference Malagelada, Barba and Accarino113). Elsewhere, in a comprehensive study examining a range of mechanisms potentially involved in the appetite response to mycoprotein compared with chicken, Bottin et al. (Reference Bottin, Swann and Cropp114) constructed orthogonal projection latent structure (OPLS) models to identify variation in metabolites in urine associated with fullness following each meal. Urinary creatinine (a breakdown product of creatine phosphate) was inversely associated with fullness following both meals. The metabolite α-keto-β-methyl-N-valerate (a deamination product of isoleucine) was also inversely associated with fullness, and β-hydroxybutyrate was positively associated with fullness following the mycoprotein but not chicken meal(Reference Bottin, Swann and Cropp114). The latter finding regarding β-hydroxybutyrate is supported by evidence from ketone ester drinks(Reference Stubbs, Cox and Evans99), highlighting one potential mechanism by which mycoprotein may suppress appetite.
In another untargeted metabolomics study of plasma pre- and post-rye bread consumption (at least 20 % of daily energy intake) over 8 weeks, 540 metabolites were profiled and specific metabolites identified that were suggested to have a role in the satiety response to rye bread(Reference Lankinen, Schwab and Seppänen-Laakso115). Among other metabolites, ribonic acid increased with rye bread consumption and was positively correlated with tryptophan – a precursor for the biosynthesis of serotonin which in turn has an impact on appetite(Reference Lankinen, Schwab and Seppänen-Laakso115). However, appetite was not measured in this study, therefore an association of these metabolites with appetite could only be speculated upon.
In a study examining appetite in female haemodialysis patients a range of endocannabinoids and fatty acids was measured by GC and liquid chromatography MS. A significant association between specific endocannabinoids and appetite measured through the Simplified Nutritional Appetite Questionnaire was reported(Reference Friedman, Kim and Kaiser116). In particular, docosatetraenoyl ethanolamide was positively correlated with appetite and a ratio of two specific endocannabinoids was inversely associated with appetite. Although the cross-sectional design prevents any causal inference, these findings nevertheless highlight a link between circulating endocannabinoids and appetite in haemodialysis patients and provide several avenues for further research in this patient population.
Overall, although limited in number, these studies highlight the potential for metabolomic approaches to deepen understanding of the complex effects of different nutrients and foods on appetite and in understanding alterations in appetite in different conditions in humans.
Methodological considerations and future directions
A role for additional metabolites
The present review has focused on metabolites that have been identified in human biofluids, and has shown direct associations with appetite and/or energy intake in humans. Several additional metabolites have been measured in different biofluids in humans and implicated to have a role in appetite control; however, often appetite was not assessed or direct associations between the metabolite concentration and appetite or energy intake were not reported. For example, in addition to bile acids, plasma acylcarnitines and phospholipid levels have been shown to change in response to bariatric surgery but associations with appetite were not determined(Reference Fiamoncini, Fernandes Barbosa and Arnoni Junior117). Plasma acylcarnitines may be a consequence of alterations in insulin resistance and metabolic flexibility(Reference Ramos-Roman, Sweetman and Valdez118,Reference Adams, Hoppel and Lok119) , rather than a marker of appetite. Additional relevant metabolites may include enterostatins which have been shown to reduce food intake, particularly from high-fat foods in rats(Reference Erlanson-Albertsson, Mei and Okada120). In women with obesity, a blunted enterostatin response has been shown after a meal(Reference Prasad, Imamura and Debata121), although appetite was not measured in that study. It should also be acknowledged that some metabolites may indirectly have an impact on appetite or provide insight into the role of other metabolites in appetite control. For example, tissue and circulating ceramides may indirectly disrupt the hypothalamic control of food intake by increasing insulin resistance(Reference Summers and Goodpaster122). In addition, as the present review is a narrative review it is possible that some relevant articles were missed. Future systematic reviews on specific metabolites or groups of metabolites may yield further information.
A role for a range of biofluids
Blood is the predominant biofluid used in studies to date, although some have studied metabolites in saliva(Reference Kong, Ferracane and De Luca50,Reference Epel, Lapidus and McEwen107) and urine(Reference Bottin, Swann and Cropp114). The latter are attractive samples to identify potential appetite biomarkers due to the non-invasive nature of collection and warrant further study. Although invasive to collect, the characterisation of metabolites in other biofluids such as cerebrospinal fluid may also offer further insights into biological processes of appetite control. For example, neurotransmitter metabolite levels in cerebrospinal fluid have been shown to differ in individuals with bulimia, with lower serotonin and dopamine metabolite concentrations compared with healthy controls(Reference Jimerson, Lesem and Kaye123). Given that the metabolite concentrations were inversely correlated with frequency of binge eating, higher concentrations of these metabolites could potentially contribute to a blunted satiety response in individuals with bulimia(Reference Jimerson, Lesem and Kaye123). The faecal metabolome largely reflects gut microbial composition(Reference Zierer, Jackson and Kastenmüller124) and therefore presents a key method of gaining further insight into the role of the gut microbiome in appetite control. Gut microbes are able to produce various metabolites which may exert their effects on appetite either directly by interacting with receptors on L-cells in the intestine or by translocating from the intestine into the peripheral circulation (for a detailed review, see van de Wouw et al. (Reference Van de Wouw, Schellekens and Dinan125)). For example, γ-aminobutyric acid (GABA), an inhibitory neurotransmitter and naturally occurring amino acid, has been identified in human faeces (as well as saliva, urine, blood and cerebrospinal fluid), and can be produced in the intestine by strains of Lactobacillus and Bifidobacterium (Reference Barrett, Ross and O’Toole126).
What influences peripheral metabolites implicated in appetite control?
The long-term molecular link between energy needs and daily intake has been commonly thought to be driven by feedback signals arising from adipose tissue such as leptin; and gut and adipose tissue derived peripheral signals have been the focus of the majority of studies investigating appetite and obesity(Reference Lean and Malkova127). However, while these signals clearly have an important role in different aspects of appetite control, other signals such as a molecular signalling pathway arising from lean tissue such as skeletal muscle may also feature(Reference Hopkins and Blundell128-Reference Grannell, De Vito and Murphy130), but to date has received little attention. Recent evidence has linked fat-free mass, RMR(Reference Blundell, Caudwell and Gibbons129,Reference Hopkins, Finlayson and Duarte131,Reference Hopkins, Finlayson and Duarte132) and activity energy expenditure(Reference Hopkins, Duarte and Beaulieu133) to the tonic drive component of appetite control that is thought to reflect biological energy requirements. However, the molecular signalling pathways that link the energy demands arising from metabolically active tissues to energy intake remain unclear. This highlights that in addition to signals arising from the gut and adipose tissue, other tissues and factors should also be considered when identifying biological signals and potential metabolites involved in appetite control.
Providing insight into individual variability in appetite and appetite responses
In addition to increasing understanding of appetite responses to ingestion of different foods, metabolomics could also have a role in increasing understanding of different appetite and behavioural phenotypes. For example, the ‘low satiety phenotype’, i.e. those with lower satiety responsiveness to a test meal, is often assessed using the satiety quotient which relates the suppression of hunger, fullness and desire to eat to the amount of energy consumed(Reference Drapeau, King and Hetherington134), and has been characterised by a tendency towards a higher anxiety level and blunted cortisol response(Reference Drapeau, Blundell and Gallant135), along with a higher level of disinhibition and greater wanting of high-fat food(Reference Dalton, Hollingworth and Blundell136), among other characteristics. Similarly, metabolomics may help to understand different binge-eating subtypes and a range of other phenotypes such as those susceptible or resistant to weight gain(Reference Dalton, Finlayson and Esdaile137).
Metabolomics could provide further insight into the wide individual variability that occurs in appetite and energy intake responses to different interventions. Some studies have classified individuals as compensators or non-compensators based on whether they ate more or less in response to exercise(Reference Hopkins, Blundell and King138,Reference King, Caudwell and Hopkins139) . Although metabolomics analysis was not conducted in these studies, baseline postprandial profiles of appetite-related peptides have been shown to identify those susceptible or resistant to exercise-induced weight loss(Reference Gibbons, Blundell and Caudwell140). Elsewhere in response to dietary intervention(Reference Fiamoncini, Rundle and Gibbons141), metabolomics analysis was carried out on seventy healthy individuals during a mixed-meal tolerance test over 8 h, and about 300 metabolites in plasma were profiled. Two distinctive ‘metabotype’ clusters were found, with only participants from one ‘metabotype’ showing positive changes in the glycaemic response after 12 weeks. Although, appetite was not assessed in this study, this highlights the potential for metabolomics to aid in understanding individual variability in responses to different interventions.
A role as part of a larger panel of appetite-related assessments
As noted for many metabolites discussed, there is covariation with other postprandial metabolites, and with the release of appetite-related gut peptides, which often mirror postprandial appetite ratings. However, the relative contribution of the different markers to appetite control is unknown. There is no composite peptide or metabolite measure that is similar to a rating of subjective appetite, raising the question of whether peptide biomarkers provide stronger evidence than subjective ratings(Reference Gibbons, Hopkins and Beaulieu33). However, while clearly biomarkers should not replace subjective ratings of appetite or actual measures of food intake, they may be extremely useful used in conjunction with a larger assessment involving a range of psychological, physiological and behavioural measures to improve mechanistic understanding behind changes in appetite and energy intake, along with other components of appetite not addressed in the present review such as food reward. An example study workflow is shown in Fig. 3.
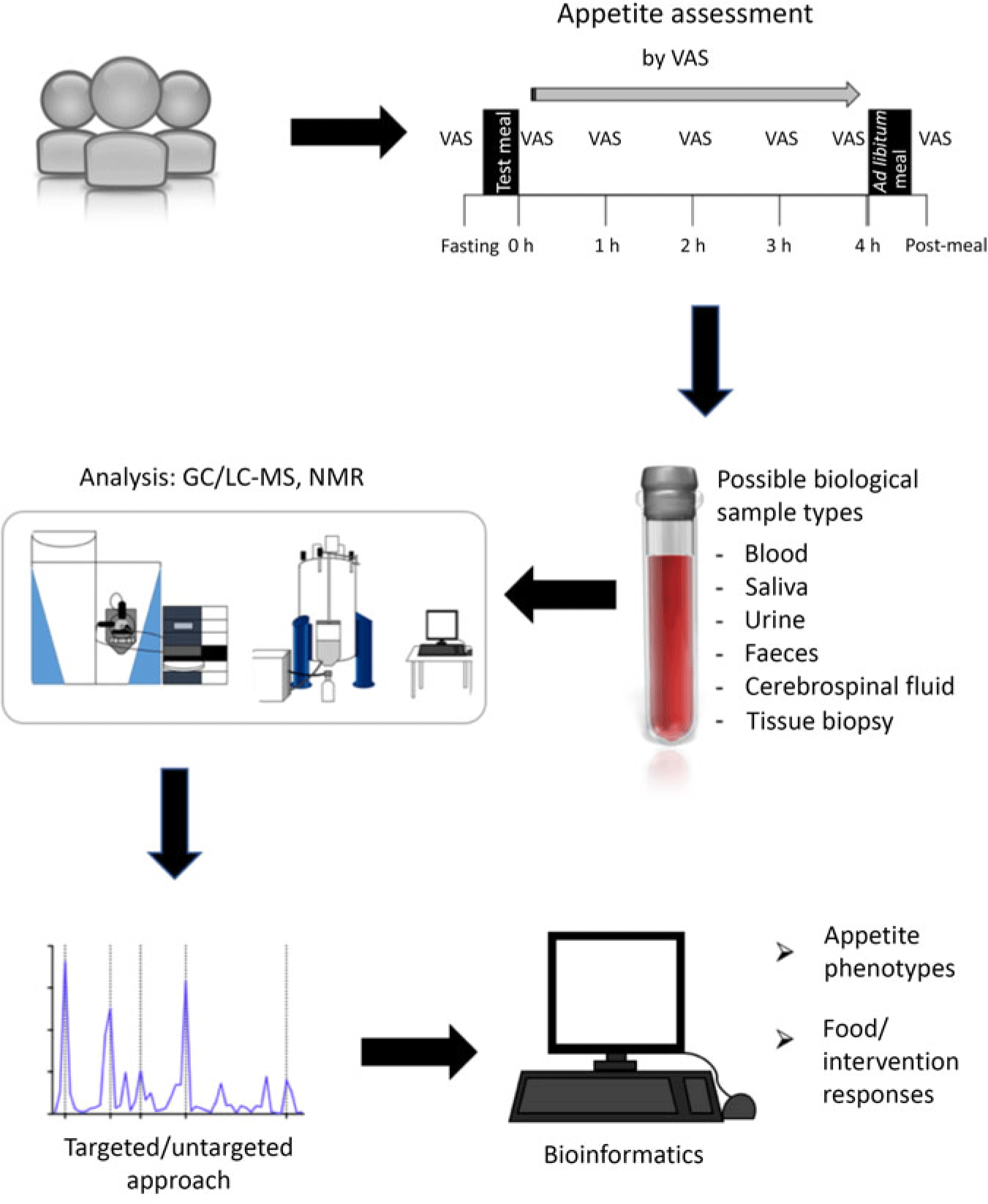
Fig. 3. Illustration of a study workflow investigating biomarkers of appetite using metabolomics. The time intervals for assessing appetite and energy intake represent one example in this illustration and will vary depending on the objectives and characteristics of a study. VAS, visual analogue scale; LC, liquid chromatography.
A role in conjunction with other ‘omics’ methods
While the present review has focused on metabolomics and relationships between metabolites and appetite ratings or energy intake, a combination of different ‘omics’ methods may also yield further information. For example, in combination with metabolomics, metagenomics and epigenomics may provide important insights into links between stress, appetite and obesity (for a comprehensive review, see Michels(Reference Michels142)). Elsewhere, proteomics analysis of plasma in the fasting state revealed apoA-IV as a putative satiety factor that rises following gastric bypass surgery and could potentially contribute to weight loss(Reference Culnan, Cooney and Stanley143). Moreover, the salivary proteome may be used to identify proteins and peptides which can be used as biomarkers of satiety(Reference Harthoorn, Schipper and Loof144). Although cost and other logistical aspects are limiting factors for widespread feasibility, to gain greater insight into underlying mechanisms and initially discover potential biomarkers, metabolomics profiles should ideally be considered alongside a range of other biological signals.
Conclusion
Appetite is a complex integrative process. In addition to environmental, psychological and behavioural factors, appetite is influenced by a range of short- and long-term biological signals. While the list of biological signals and potential biomarkers of appetite in humans is continuing to increase, many metabolites including glucose and amino acids were proposed as key signalling molecules in appetite over 60 years ago. Some metabolites appear to be associated with appetite under specific conditions, such as in energy deficit, or be dependent on concentrations of other metabolites such as glucose. However, while several studies to date show associations between circulating metabolites and appetite, in many cases causal relationships in humans remain to be established, highlighting the need for longitudinal studies. Furthermore, many studies have targeted single metabolites or a limited number of metabolites and/or gut peptides. Modern metabolomics facilitates the measurement of hundreds of metabolites using a targeted and/or untargeted approach, and has significant potential to identify potential biomarkers and deepen understanding of the complex biological signals involved in appetite control. This could in turn aid in improving strategies targeting appetite control and in tailoring strategies more effectively to individuals.
Acknowledgements
No sources of funding were received for the present review.
K. H. and L. B. contributed to the conception and design of the review. K. H. and L. B. drafted the initial manuscript. K. H., M. H., C. G., G. F. and L. B. reviewed and edited the manuscript.
There are no conflicts of interest to declare.








