Introduction
Chronic rhinosinusitis is a common condition affecting millions of people worldwide.Reference Albu1 It is a diverse condition with different phenotypes. There are two main phenotypes of chronic rhinosinusitis: chronic rhinosinusitis with and without nasal polyps.Reference Cho, Hamilos, Han and Laidlaw2 It is estimated that only 25–30 per cent of patients with chronic rhinosinusitis have nasal polyps.Reference Stevens, Schleimer and Kern3 However, those with chronic rhinosinusitis with nasal polyps struggle with worse symptoms and quality of life.Reference Stevens, Schleimer and Kern3
Although understanding phenotypes is important, recent advances in understanding the endotypes of this disease have broadened the treatment options available. The disease endotype explains the pathophysiology behind it. This allows the use of targeted therapies that interfere with disease progression.Reference Brescia, Zanotti, Parrino, Barion and Marioni4 The pathophysiology of chronic rhinosinusitis with nasal polyps has been investigated, and it is known to be characterised by type 2 inflammation, where there are high levels of eosinophils and type 2 cytokines. Although there may be some overlap with type 1 and type 3 inflammation, the frequency of type 2 inflammation was found to be significantly higher in chronic rhinosinusitis with nasal polyps compared to chronic rhinosinusitis without nasal polyps (p < 0.001).Reference Stevens, Peters, Tan, Klingler, Poposki and Hulse5
Immunology of type 2 inflammation
The type 2 inflammatory response is associated with elevated levels of interleukin (IL)-4, IL-5, IL-13, IL-25 and IL-33.Reference Chen and Buchheit6 IL-5 has an important role in eosinophils formation and survival.Reference Gevaert, Han, Smith, Sousa, Howarth and Yancey7,Reference Simon, Yousefi, Schranz, Schapowal, Bachert and Blaser8 IL-5 has been found to upregulate eosinophilic CD69 expression;Reference Soman, Stafford, Pazdrak, Wu, Luo and White9 in addition, higher levels of IL-5 and IL-13 are associated with more severe chronic rhinosinusitis with nasal polyps.Reference Chen and Buchheit6,Reference Tomassen, Vandeplas, Van Zele, Cardell, Arebro and Olze10 IL-4 and IL-13 cause goblet cell hyperplasia, tissue fibrosis, and induce tissue remodelling in patients with eosinophilic disorders.Reference Chen and Buchheit6,Reference Doran, Cai, Holweg, Wong, Brumm and Arron11 Table 1 shows a summary of important cytokines involved in type 2 inflammation.Reference Gevaert, Han, Smith, Sousa, Howarth and Yancey7,Reference Simon, Yousefi, Schranz, Schapowal, Bachert and Blaser8,Reference Kato12–Reference Baba, Kondo, Kanaya, Suzukawa, Ushio and Urata17 Various studies have also mentioned tissue remodelling in patients with chronic rhinosinusitis with nasal polyps. This can be attributed to reduced levels of collagen and fibrin deposition, which may be induced by IL-13.Reference Kato12
Table 1. Cytokines involved in type 2 inflammatory process and their role in Chronic rhinosinusitis with nasal polyps
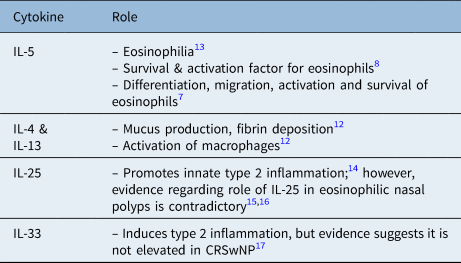
CRSwNP = chronic rhinosinusitis with nasal polyps; IL = interleukin
Eosinophilic chronic rhinosinusitis with nasal polyps is commonly seen in Western countries; however, there is also another subtype of chronic rhinosinusitis with nasal polyps that is not characterised by eosinophilia and which is more recognised in Asian countries, including Japan, Korea and China.Reference Yao, Zeng and Liu18,Reference Wang, Zheng, Liu and Guo19 Nevertheless, recent studies conducted in Korea and Thailand have shown a recent increase in eosinophilic nasal polyps in Asia.Reference Kato12
Current treatment guidelines
Current guidelines recommend the use of various medical treatments in a stepwise approach. This includes saline irrigation, steroid sprays or drops, and systemic steroid courses.20 If medical treatment fails to control the symptoms, then surgical intervention would be appropriate.20 A cross-sectional study conducted in the UK found that 46 per cent of patients with chronic rhinosinusitis with nasal polyps reported having more than one surgical polypectomy performed.Reference Philpott, Hopkins, Erskine, Kumar, Robertson and Farboud21 Another study found that up to 78.9 per cent of patients with chronic rhinosinusitis with nasal polyps reported disease recurrence after functional endoscopic sinus surgery (FESS).Reference Calus, Van Bruaene, Bosteels, Dejonckheere, Van Zele and Holtappels22 These findings reflect the burden of chronic rhinosinusitis with nasal polyps and the importance of finding more effective treatment modalities.
Biological products, particularly monoclonal antibodies, have revolutionised the treatment of auto-immune diseases and some cancers.Reference Lu, Hwang, Liu, Lee, Tsai and Li23 They also have a role in treating patients with chronic rhinosinusitis with nasal polyps considering the role of type 2 inflammation, and the role of IL-4 and IL-13 (discussed above) in chronic rhinosinusitis with nasal polyps progression and polyp formation. Dupilumab, a novel monoclonal antibody that has recently been approved for the treatment of chronic rhinosinusitis with nasal polyps, shows promising results in different studies.Reference Calus, Van Bruaene, Bosteels, Dejonckheere, Van Zele and Holtappels22 This paper describes a literature review conducted to compare FESS and biological therapy for the management of patients with chronic rhinosinusitis with nasal polyps. The main objectives were to compare the two treatment modalities in terms of quality of life, costs and complications.
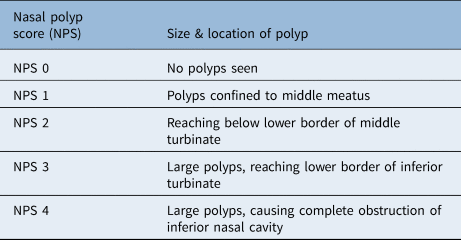
Different scoring systems were used in the papers reviewed. The 22-item Sinonasal Outcome Test (SNOT-22) is a questionnaire used to assess sinonasal symptoms. It includes five domains, including physical and psychological symptoms, and gives a total score of 0–110, with 110 being the most severe.Reference Khan, Reaney, Guillemin, Nelson, Qin and Kamat24 Other studies used quality-adjusted life years. According to the National Institute for Health and Care Excellence, ‘One quality-adjusted life year is equal to 1 year of life in perfect health’.25 Table 2 shows the nasal polyp scoring system, which was also utilised in the papers reviewed.Reference Miglani, Soler, Smith, Mace and Schlosser26
Table 2. Nasal polyp scoring system
Materials and methods
Three databases, PubMed, ScienceDirect and Cochrane, were used to obtain relevant papers for this review. EndNote was then used to remove duplicate papers during the selection process. Figure 1 shows the number of papers obtained from each database. The search was conducted from April 2023 to May 2023. One reviewer (AA) conducted the search, and screened the papers by title and abstract during the selection process. Table 3 shows the features of the papers, reviewed in chronological order.Reference Miglani, Soler, Smith, Mace and Schlosser26–Reference van der Lans, Fokkens, Adriaensen, Hoven, Drubbel and Reitsma31 Data collection was conducted manually by a single reviewer (AA). A data extraction table was used for data collection, containing three main domains: symptom relief or quality of life, cost-effectiveness, and complications.
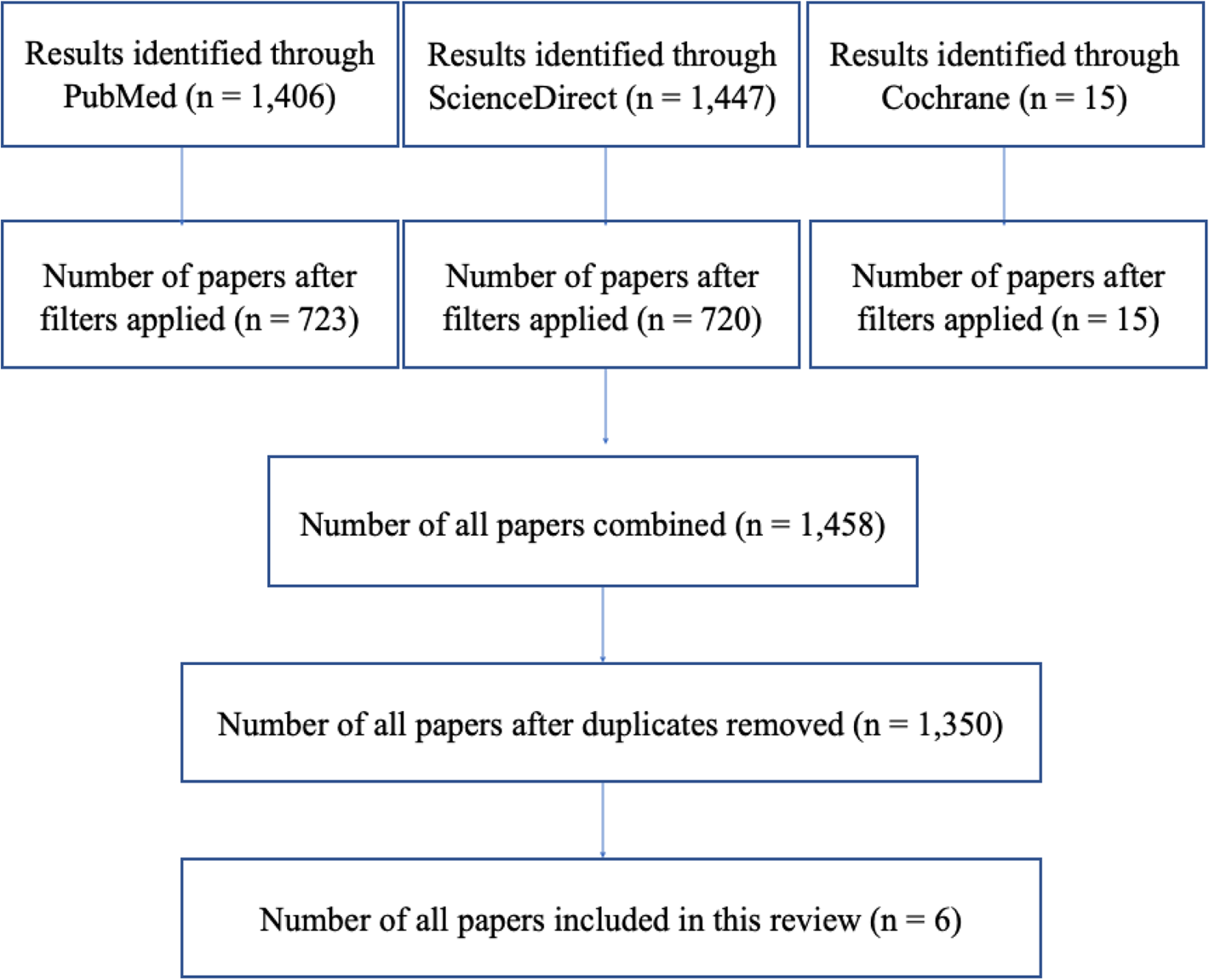
Figure 1. Literature review flow chart.
Table 3. Features of the papers reviewed
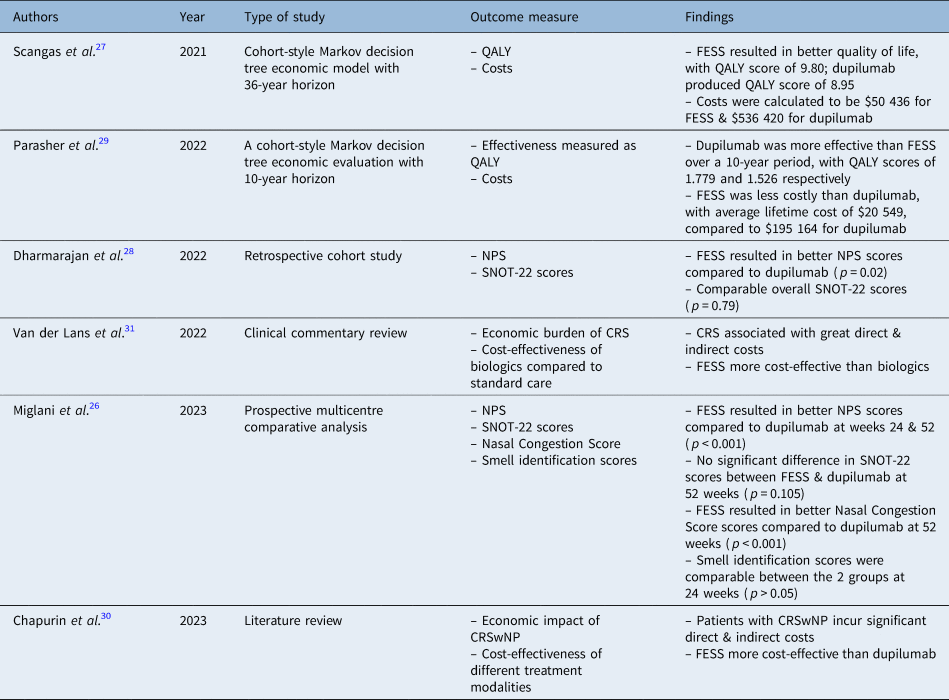
QALY = quality-adjusted life year; FESS = functional endoscopic sinus surgery; NPS = nasal polyp scores; SNOT-22 = 22-item Sinonasal Outcome Test; CRS = chronic rhinosinusitis; CRSwNP = chronic rhinosinusitis with nasal polyps
Results
Symptom relief, quality of life and sinonasal outcome
With regard to symptom relief and quality of life, varying results have been reported in the literature. The cohort-style Markov model by Parasher et al. found dupilumab to be more effective than FESS, with reported quality-adjusted life year scores of 1.779 and 1.526 respectively.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 However, the cohort-style Markov model by Scangas et al. reported a higher quality-adjusted life year score in the FESS group, of 9.80, compared to 8.95 in the dupilumab group.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27
In terms of SNOT-22, a general improvement was reported with both treatment modalities in all papers identified, and there was a consensus that overall SNOT-22 scores were comparable (Dharmarajan et al.Reference Dharmarajan, Falade, Lee and Wang28 p = 0.79, and Miglani et al.Reference Miglani, Soler, Smith, Mace and Schlosser26 p = 0.105). Dharmarajan et al. reported better improvement in the psychological SNOT-22 domain in the FESS group (p = 0.03).Reference Dharmarajan, Falade, Lee and Wang28 However, the dupilumab group had better extranasal and olfaction scores (p = 0.02 and p = 0.04 respectively).
Nasal polyp score
The study conducted by Dharmarajan et al. reported that FESS was superior to dupilumab in reducing nasal polyps, as a bigger reduction in nasal polyp score was seen in the FESS group (mean score of 5.18 ± 2.01) compared to the dupilumab group (mean score of 4.27 ± 1.98) (p = 0.02).Reference Dharmarajan, Falade, Lee and Wang28 Miglani et al. reported a similar significant improvement in nasal polyp score in the FESS group compared to all biologics at 24 and 52 weeks (p < 0.001).Reference Miglani, Soler, Smith, Mace and Schlosser26
Costs
According to Parasher et al., the costs for dupilumab over a 10-year period were $195 164 compared to $20 549 for the FESS group.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 A similar cost trend was found by Scangas et al., who reported a total cost of $50 436 for FESS compared to $536 420 for dupilumab over a period of 36 years.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27
Discussion
Almost all of the papers reviewed compared the quality of life of the two cohorts post treatment. Two studies used the SNOT-22 questionnaire to quantify the effect of FESS and biologics on symptom control. Two studies used quality-adjusted life years to quantify the effect of the interventions on patients’ quality of life. Two studies compared the cost-effectiveness of the two treatments. However, the papers reviewed did not mention the complications of the treatments studied.
Although the papers included in this review did not include the complications, it is important to note that both medical and surgical treatment options can result in several complications. A retrospective cohort analysis of 78 944 patients who underwent FESS reported a major complication rate of 0.36 per cent in primary cases and a rate of 0.42 per cent following revision surgery.Reference Krings, Kallogjeri, Wineland, Nepple, Piccirillo and Getz32 Dupilumab was first approved by the US Food and Drug Administration in 2019 for the treatment of chronic sinusitis with nasal polyps.33 There are several reported complications associated with the use of dupilumab; for instance, a randomised controlled trial reported that 47 per cent of the treatment group developed nasopharyngitis, 20 per cent developed a headache and 40 per cent reported a reaction at the injection site.Reference Bachert, Mannent, Naclerio, Mullol, Ferguson and Gevaert34
Nasal polyp and questionnaire scores
Chronic rhinosinusitis has a profound effect on patients’ quality of life and well-being. In addition to rhinological symptoms, other debilitating symptoms have been reported. These include poor quality of sleep, fatigue and poor cognitive function.Reference DeConde and Soler35 These profound symptoms emphasise the importance of finding appropriate treatments to improve patients’ quality of life. Hence, one of the primary outcomes of this literature review concerns the effect of the two treatment types on patients’ quality of life.
Nasal polyp scores were significantly better in the FESS group compared with the dupilumab group in the studies conducted by Dharmarajan et al.Reference Dharmarajan, Falade, Lee and Wang28 and Miglani et al.Reference Miglani, Soler, Smith, Mace and Schlosser26 This is not surprising as surgical resection of the polyps would confer immediate results compared to medical management of any type. Although nasal polyp score is an important indicator of treatment effectiveness, the ultimate treatment goal should be patients’ satisfaction and quality of life.
The studies conducted by Dharmarajan et al.Reference Dharmarajan, Falade, Lee and Wang28 and Miglani et al.Reference Miglani, Soler, Smith, Mace and Schlosser26 reported comparable improvement in SNOT-22 scores in both treatment modalities. However, FESS resulted in better scores in the psychological domain of the SNOT-22 questionnaire. It is well established that patients tend to expect better results with surgery, and several trials have reported improved symptoms following placebo interventions.Reference Jonas, Crawford, Colloca, Kaptchuk, Moseley and Miller36 While FESS is not a placebo surgery, and the improved psychological symptoms might not be related to the mode of treatment, a more invasive mode of treatment, including placebo surgery, has been reported to improve symptoms in patients.Reference Wartolowska, Judge, Hopewell, Collins, Dean and Rombach37
According to Dharmarajan et al.,Reference Dharmarajan, Falade, Lee and Wang28 dupilumab achieved better improvement in the extranasal rhinological domain compared to FESS (p = 0.02) and achieved better olfaction scores (p = 0.04). This may be because dupilumab works systemically, and its effect is not limited to shrinking nasal polyps; thus, it has therapeutic effects on cough and nasal discharge. Better olfaction scores in the dupilumab arm is also consistent with the literature, as improvement in smell scores following dupilumab therapy has been previously reported.Reference Mullol, Bachert, Amin, Desrosiers, Hellings and Han38 These findings are helpful to tailor therapy around patients’ main symptoms and SNOT-22 scores, as patients with worse extranasal SNOT-22 scores, or whose loss of smell is their main complaint, might be good candidates to receive dupilumab therapy. However, Miglani et al. reported comparable changes in smell identification between the two treatment modalities at 24 weeks; unfortunately, no comparison was made at 52 weeks because of unavailable data.Reference Miglani, Soler, Smith, Mace and Schlosser26
Taking these findings into account, although FESS resulted in better nasal polyp scores, the overall SNOT-22 scores were comparable between the two treatments, suggesting that they are equally effective.
Quality-adjusted life year scores
The two studies comparing dupilumab and FESS in terms of quality-adjusted life year scores produced contradictory results.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27,Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 According to Parasher et al., patients who did not respond to dupilumab were then assigned to FESS.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 This means that patients with treatment-resistant disease might eventually have undergone FESS, which would explain the higher quality-adjusted life year scores reported for patients in the dupilumab arm. Furthermore, dupilumab is a systemic therapy and can also improve symptoms of asthma and atopic dermatitis,Reference Tameez Ud Din, Malik, Arshad and Tameez Ud Din39,Reference Bachert, Han, Desrosiers, Hellings, Amin and Lee40 which will result in improved quality-adjusted life year scores overall. Although Parasher et al. reported better quality-adjusted life year scores in the dupilumab group, it was estimated that approximately 40 years of dupilumab treatment is needed to achieve a full one-unit quality-adjusted life year difference.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 Hence, the higher quality-adjusted life year scores achieved by dupilumab may be negligible considering how challenging it is to sustain 40 years of continuous therapy in clinical practice.
Another factor that might explain the discrepancy in quality-adjusted life year scores between the two studies is the different cohorts,Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27,Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 and Scangas et al. included a sample from a single centre.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27 Scangas et al. reported better quality-adjusted life year scores in the FESS group (score of 9.80) compared to the dupilumab group (score of 8.95).Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27 However, the medical treatment post-operatively was not standardised; thus, if the surgeon had prescribed more aggressive medical treatment, this would subsequently have improved reported quality-adjusted life years in the FESS group.
Although using quality-adjusted life year scores can provide useful information, there are a few limitations to consider. The quality-adjusted life year is often used for chronic diseases; however, patients’ views on health may change throughout the disease course, and, in patients with chronic rhinosinusitis with nasal polyps, there might be other co-morbidities affecting quality-adjusted life year scores.Reference Rudmik and Drummond41
Cost-effectiveness
Cost-effectiveness is an important factor to consider when choosing a treatment modality. A comparison between medical versus surgical management has been previously performed, and it was concluded that FESS is more cost-effective than medical management (biologics not included).Reference Chapurin, Khan, Gutierrez and Soler30
The cost-effectiveness of biologics and FESS was compared by Scangas et al.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27 and Parasher et al.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 Both papers concluded that FESS is a significantly more cost-effective option than dupilumab. Parasher et al. reported a total cost of $20 549 for FESS and $195 164 for dupilumab therapy over a period of 10 years.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 Although dupilumab was found to generate better quality-adjusted life years than FESS in that study, the difference in cost was astonishing, and the annual costs of dupilumab would have to be reduced to $1870 to achieve the same cost-effectiveness of surgery.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29
Similar results were reported in the paper by Scangas et al.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27 It was found that the FESS costs, over a 36-year period, were around $50 436, compared to $536 420 in the dupilumab group. The papers by Scangas et al.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27 and Parasher et al.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 calculated the annual cost of dupilumab at a price of $31 000 per year; however, the study by Parasher et al. did not account for the costs of dupilumab complications.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 The differences in the costs reported by the two papers can be explained by the difference in the time horizon of the two studies, as Parasher et al. used a 10-year time horizon,Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 while Scangas et al.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27 used a 36-year model. Although the comparison by Scangas et al.Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27 might be more accurate, as the costs of dupilumab complications were included in the analysis, a 10-year model is more representative and relevant to the current market, as the price of dupilumab is very likely to change after 10 years.
The findings of both studies mentioned aboveReference Scangas, Wu, Ting, Metson, Walgama and Shrime27,Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 suggest that FESS is far more superior in terms of cost-utility. However, there are a few points to be considered when assessing the cost-effectiveness of dupilumab. The drug patent for dupilumab is due to expire in the next 10 years, and the drug will be available at a far more affordable price, which would necessitate reanalysing the cost-effectiveness of this treatment.42,43 The ‘LIBERTY NP SINUS-52’ clinical trial investigated increasing the dose interval from 300 mg every 2 weeks to 300 mg every 4 weeks, after an initial 24-week course and sustained good clinical outcomes.Reference Bachert, Han, Desrosiers, Hellings, Amin and Lee40 This suggests that after an interim period of at least 24 weeks, costs of dupilumab may be cut in half by increasing the dose interval in a stepwise approach. Another study trialled increasing the dose interval following the same initial period of 24 weeks; the dose interval was increased up to 6 weeks and good disease control was maintained.Reference van der Lans, Fokkens, Adriaensen, Hoven, Drubbel and Reitsma31 This approach can enhance the cost-effectiveness of the drug, while maintaining good disease control and preventing disease flare-ups.
Suggestions for further research
No papers included in this literature review were without a bias of one form or another. The six articles chosen for inclusion had the fewest biases, but there were no papers focusing or reporting on complications of biological therapy for chronic rhinosinusitis management.
Although the investigation conducted by Dharmarajan et al. provides useful information, it is a retrospective cohort study comprising a total of 108 participants only.Reference Dharmarajan, Falade, Lee and Wang28 The retrospective nature of that study is associated with several limitations, including selection bias. While participants were matched on baseline characteristics including asthma status, different patients might have had different methods of asthma control, which could have subsequently affected their SNOT-22 scores. There is also no way to confirm whether all patients received a standardised course of corticosteroids. The surgical group had a lower number of prior FESS procedures (p < 0.001). This could mean that the surgical group had milder disease severity. However, the FESS group had a higher Lund–Mackay score compared to the dupilumab group (p < 0.02), so the past surgical history may not reflect disease severity. Follow-up time also varied between the two groups.
The prospective study conducted by Miglani et al. confirmed there was no difference between the cohorts in terms of patient characteristics.Reference Miglani, Soler, Smith, Mace and Schlosser26 However, there was a different selection criterion for the biologics cohort and the FESS cohort, resulting in a failure of standardisation. However, it is imperative to highlight that the patients had comparable baseline Lund–Mackay computed tomography scores in both cohorts. Another limitation is that patients in the FESS group had different peri-operative treatment regimens, and this inconsistency might have affected the treatment outcomes.
The data included in Parasher et al. are derived from a trial, and there was no comparison of cohort demographics or co-morbidities.Reference Parasher, Gliksman, Segarra, Lin, Rudmik and Quast29 In the study conducted by Scangas et al.,Reference Scangas, Wu, Ting, Metson, Walgama and Shrime27 the patients were not randomised, and the FESS group chose surgical management; thus, selection bias is a possibility. The paper did not consider that some patients on dupilumab may eventually need surgical intervention, and it failed to take inflation prices into account. It also calculated the cost of dupilumab taken indefinitely; however, recent literature has shown that the treatment dose interval can be altered.Reference van der Lans, Fokkens, Adriaensen, Hoven, Drubbel and Reitsma31
While it is not possible to conduct a randomised double-blinded study,Reference Miglani, Soler, Smith, Mace and Schlosser26 the observational and Markov model studies remain the best to provide insight into this topic. The authors would implore future researchers to describe not only the benefits gained from biological treatment for chronic rhinosinusitis, but also to report on any complications attributable to this treatment modality.
Conclusion
Dupilumab, an interleukin (IL)-4 and IL-13 inhibitor, provides comparable symptom relief to FESS in patients with chronic rhinosinusitis with nasal polyps. Although FESS has been associated with better polyp scores than dupilumab, the latter is still an effective medication that shows promising results.
In terms of cost-effectiveness, FESS is far superior to dupilumab given the high costs of this biological product. This might change after the next 10 years when the patent of this drug expires. If this drug is available at a more affordable price, it will likely revolutionise the treatment of chronic rhinosinusitis with nasal polyps.
As the papers included in this review did not compare FESS with dupilumab in terms of complications, more studies with a bigger number of participants are needed to investigate this, as it will help patients and clinicians make an informed decision when considering treatment options.
Competing interests
None declared








