Iodine is an essential micronutrient required for the production of thyroid hormones, which regulate metabolism, growth and development(Reference Andersson and Braegger1). Pregnant and lactating women have higher iodine requirements because they need to support the iodine needs of their fetus and baby(Reference Bath, Verkaik-Kloosterman and Sabatier2). A recent meta-analysis reported that the overall worldwide prevalence of maternal iodine deficiency was 53 %(Reference Patriota, Lima and Nilson3). As a result, insufficient iodine intake can cause many iodine deficiency disorders, which affect both mothers and infants, e.g. congenital hypothyroidism, intellectual disability, neonatal hypothyroidism, hyperthyrotropenemia and growth retardation(Reference Brough4).
The WHO recommends assessing iodine status based on urinary iodine concentration (UIC) for non-pregnant women, defined as moderate-to-severe iodine deficiency (UIC 0–49 μg/l), mild iodine deficiency (UIC 50–99 μg/l), iodine sufficiency (UIC 100–199 μg/l) and more-than-adequate and excessive iodine status (UIC ≥ 200 μg /l)(5). In non-pregnant women, UIC is used to determine iodine status based on the principle that approximately 90 % of ingested iodine is excreted in the urine(Reference Zimmermann and Andersson6). However, using UIC to assess iodine status has some limitations. First, UIC is a short-term biomarker, which can be easily affected by recent iodine intake from the diet(Reference Zimmermann7). Second, there is high intra- and inter-individual variation in UIC(Reference Zimmermann7,Reference Als, Helbling and Peter8) . In lactating women, there is little evidence that supports the recommendation of a median UIC cut-off of < 100 μg/l to indicate iodine deficiency because iodine is also excreted in breast milk and thus < 90 % of ingested iodine is excreted via urine(Reference Brough4,Reference Andersson, De Benoist and Delange9) .
Breast milk iodine concentration (BMIC) has been reported to be a promising biomarker of iodine status in breast-feeding women(Reference Dold, Zimmermann and Aboussad10), although there is no scientific consensus on whether BMIC can accurately reflect iodine status in breast-feeding women(Reference Brough4). This is because the iodine content in breast milk is influenced by the mother’s iodine intake and overall iodine status(Reference Brough4). In addition, BMIC is independent of maternal fluid intake(Reference Brough4). A recent systematic literature review has suggested that there have been inconsistencies in the relationship between BMIC and UIC in lactating women, which may be due to the differences in lactation stages, maternal iodine status and sampling collection time(Reference Liu, Sharp and Villanueva11). Most of these studies were cross-sectional in design, and there has been a lack of high-quality, well-designed studies(Reference Liu, Sharp and Villanueva11). Therefore, WHO suggested a number of research priorities including the assessment of iodine status during pregnancy and early infancy, which include the usefulness of BMIC(Reference Andersson, De Benoist and Delange9).
To date, there is limited information regarding BMIC in breast-feeding women and on the changes of iodine status in pregnant women who are then followed until lactation. China has eliminated iodine deficiency disorders for more than 20 years and considered an iodine sufficient country based on the median UIC of studies involving school-aged children and non-pregnant adults(Reference Sun, Codling and Chang12). However, these findings may not reflect the iodine status of women during pregnancy and lactation, who, as stated earlier, have a substantially higher iodine requirement(Reference Zimmermann, Marriott, Birt and Stallings13). Some women during pregnancy and lactation remain at risk of iodine deficiency(Reference Ding, Indayati and Basnet14). Therefore, the aim of our study was to assess iodine concentration in breast milk and urine samples in Chinese breast-feeding women from Shaanxi province, an iodine sufficient region.
Methods
Study population
The Women and Iodine Nutrition study was designed as a prospective, longitudinal, observational cohort study spanning from the third trimester of pregnancy to the first week of lactation, with a follow-up period of up to 3 months, in Shaanxi Province (in the western part of China). Pregnant women were recruited between May 2021 and May 2022 at Xianyang Central Hospital Affiliated with the Medical Department of Xi’an Jiaotong University.
Inclusion criteria were as follows: pregnant women during their third trimester (gestation weeks of 28 and above); aged between 18 and 50 years; healthy, not had medically diagnosed thyroid disease, not taking thyroid medication; singleton birth; intended to breastfeed for at least 7 days; of Chinese nationality; must live in Shaanxi at least one year; able to read and write in Chinese; had a healthy, singleton, full-term birth (pregnancy weeks 38–42); infant exclusively breastfed. Infants with fetal abnormality were excluded.
Written informed consent was obtained from all eligible women. Infants were consented by their participating mothers. Ethical approval for this study was approved by the Xi’an Jiaotong-Liverpool University Ethics Committee (reference no. 20-01-09) and Xianyang Central Hospital Affiliated to the Medical Department of Xi’an Jiaotong University (reference no. 20200009). The results of the Women and Iodine Nutrition study were reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines for the cohort studies(Reference Vandenbroucke, von Elm and Altman15,Reference Von Elm, Altman and Egger16) .
Socio-demographic data collection and other maternal and neonatal data
Women were required to complete a fifty-seven-item questionnaire including a validated thirty-three-item iodine-specific FFQ (including milk products, yoghurt, eggs, seafoods and fish) and questions on socio-demographics twice (during pregnancy and lactation). The questionnaire has been previously validated and used in assessing maternal iodine status of the Chinese population(Reference Yu, Zheng and Zheng17). Other socio-demographics of participants including age, marital status, ethnicity, education level, occupation and pre-pregnancy BMI were obtained during routine antenatal care. Women who graduated high school, junior high school and elementary school and below were categorised as ‘below university level’ and those who graduated university or junior college and above were ‘university level and above’. The daily iodine doses during pregnancy and lactation were retrieved using the brand name of the supplement provided via the questionnaire used. Pre-pregnancy height and weight were recorded using a stadiometer to the closest 0·1 cm and 0·01 kg. Pre-pregnancy BMI was categorised according to the criteria by the Chinese adults proposed by the WHO’s recommendation as follows: underweight, < 18·5 kg/m2; normal weight, 18·5–24·9 kg/m2; overweight, ≥ 25·0 kg/m2 and obese, ≥ 30·0 kg/m2(18).
Data on blood pressure, thyroid-stimulating hormone, free thyroxine (FT4) and thyroid peroxidase antibody (TPOAb) during pregnancy were retrieved from medical records. The normal reference ranges for thyroid-stimulating hormone, FT4 and TPOAb were as follows: 0·27–4·20 µIU/ml, 0·93–1·70 ng/dl and < 34·0 μg/ml. Thyroid-stimulating hormone, FT4 and TPOAb values were determined using electrochemiluminescence immunoassay on a Roche E602 immunochemistry analyser(Reference Li, Lu and Si19). In addition, total gestation weeks at delivery, sex, type of delivery, birth weight and length and APGAR score (appearance, pulse, grimace, activity and respiration) at 1, 5 and 10 min were also assessed and obtained from neonatal records.
Urine sample collection
During the third trimester of pregnancy, participants were provided with instructions and equipment to collect one approximately 20 ml (non-fasting), mid-stream urine sample between 09.00 and 12.00. During the first week of lactation, participants were asked to provide two (non-fasting), mid-stream urine samples between 09.00 and 12.00 on two separate days (i.e. the 3rd and 4th day of lactation period), to control for the intra-individual and within-day UIC variation, while minimising participant burden(Reference Mulrine, Skeaff and Ferguson20). The internationally recommended method to determine iodine status based on UIC is the collection of morning or spot urine samples in the non-fasting state(5).
Breast milk sample collection
Women were asked to clean their breasts with water before collecting the breast milk sample manually. Approximately one 5 ml breast milk sample (non-fasting sample) was collected between 09.00 and 12.00 from women before their infants were fed at the 3rd day of lactation because not all women’s milk would come in on the 1st or 2nd day of lactation(Reference Mulrine, Skeaff and Ferguson20,Reference Jorgensen, O’Leary and James21) . Since the literature review does not show whether BMIC is affected by a recent meal, women were allowed to have their usual diet before breast milk collection(Reference Semba and Delange22). Currently, there is no evidence supporting the fact that BMIC differs with regard to the milk from the start and end of a feed, left or right breast or diurnal variation(Reference Andersen, Møller and Laurberg23,Reference Dorea24) .
Laboratory analysis
All urine and breast milk aliquots were kept frozen at −20°C from time of sampling in Xianyang Central Hospital Affiliated to the Medical Department of Xi’an Jiaotong University until analysis.
After thawing, samples were vortexed using a vortex mixer until homogenous. Samples from the same woman were analysed in the same batch.
Assessment of breast milk iodine concentration and urinary iodine concentration in lactating women
BMIC and UIC were measured colourimetrically based on the Sandell–Kolthoff reaction adapted for a 96-well microplate using a microplate reader (Denley Dragon, Wellscan MK 3, Thermo Fisher Scientific)(Reference Yan, Zhang and Liu25). The equipment was calibrated following the manufacturer’s instructions and quality control samples with known concentrations of iodine were included in the same run of the iodine analysis for both BMIC and UIC. A BMIC reference range of 60–465 μg/l is used as indicative of sufficient iodine status in exclusively breast-feeding women residing in iodine sufficient regions(Reference Dold, Zimmermann and Aboussad10). The recommended UIC cut-offs during pregnancy and lactation to determine iodine sufficiency are ≥ 150 and ≥ 100 µg/l, respectively(5).
Statistical analysis
SPSS statistical software package version 25·0 (IBM Corp.) was used to perform the statistical analysis. Parametric data were expressed as mean and standard deviation, and non-parametric data were presented as median (25th, 75th percentile). Categorical variables were reported as counts and percentages, χ 2 tests were used to assess differences in categorical variables. For related samples, comparisons of medians or means were performed using Wilcoxon sign rank tests for non-normally or paired t-test was used for normally distributed variables.
Receiver operating curves were constructed to determine the diagnostic performance of BMIC using a UIC cut-off of 100 ug/l for lactating women. An optimal cut-off for BMIC was identified and sensitivity (proportion of cases correctly identified), specificity (proportion of non-cases correctly identified) along with negative predictive value and positive predictive value were then calculated to assess the accuracy of the cut-off(Reference Li and He26,Reference Parikh, Mathai and Parikh27) . Sensitivity is defined as the proportion of those who are correctly identified as iodine deficient by BMIC (true positives), while specificity is defined as the proportion of those who are correctly identified as not iodine deficient by BMIC (true negatives)(Reference Li and He26,Reference Parikh, Mathai and Parikh27) . The negative predictive value is expressed as the proportion of those with negative test results who are correctly identified as not iodine deficient. The positive predictive value is reported as the proportion of those with positive test results who are correctly identified as iodine deficient(Reference Li and He26,Reference Maxim, Niebo and Utell28) . As there is no gold standard to assess individual iodine status for lactating women, the mean of two spot urine samples collected from each lactating women was used as the reference standard for the determination of the sensitivity, specificity, negative predictive value and positive predictive value of BMIC. When the area under the receiver operating curve is ≥ 0·7, it is considered to have the acceptable discrimination for distinguishing iodine deficiency from iodine sufficient(Reference Hosmer, Lemeshow and Sturdivant29).
A correlation between BMIC and maternal UIC was assessed using a Spearman correlation coefficient. Logistic regression analysis was used to assess the associations between the predictors of BMIC and maternal UIC with adjustment for covariates. The dependent variables were BMIC and maternal UIC. Covariates adjusted in regression models included variables of age, UIC pregnancy, UIC lactation, delivery type, occupation and education. A P < 0·05 was taken as level of significance.
For the calculation of 24-h breast milk iodine excretion, a breast milk volume of 0·8 l/d was used(Reference Andersson and Braegger1,Reference Russell, Beard and Cousins30) . The estimated infant’s iodine intake is calculated as follows: total volume of breast milk consumed by the infant multiplied by BMIC. Currently, there is no recommended iodine intake for infants aged < 1 month old(Reference Dold, Zimmermann and Baumgartner31).
The primary outcome of this study was BMIC, and the sample size was calculated using G * Power 3·1 (Heinrich Heine University) based on data (mean and standard deviation) from a study of Chinese lactating women(Reference Wang, Sun and Zhang32). Therefore, on the basis of the literature and using BMIC as the primary outcome, in order to detect an effect size of 0·5, with 90 % power and two-sided alpha (0·05), this meant at least eighty women would be needed in each group to detect a significant difference between the iodine deficient and iodine sufficient groups. After accounting for 20 % attrition, a final sample size of 192 women was needed for the whole study.
Results
Study population
Of the 227 women who were approached and invited to participate, 200 pregnant women fulfilled the study criteria and were enrolled in the study. Those who were excluded from the study were either ineligible for the study criteria (n 3) or not interested in the study (n 24). Table 1 summarises the basic characteristics of women and their infants included in the study. The mean age of the women was 29·0 ± 4·2 years. The study population consisted of pregnant women with gestational ages ranging from 29 weeks and 6 days to 40 weeks and 3 days, with a mean gestational age of 37 weeks. All women were negative for TPOAb. The mean pre-pregnancy weight of the participants was 55·9 ± 8·4 kg, who had a mean weight gain of 14·9 ± 3·7 kg during pregnancy. Their mean systolic blood pressure was 115·1 ± 10·5 mm Hg, and their mean diastolic blood pressure was 74·9 ± 7·3 mm Hg. Only 1·5 % of participants (n 3) were smokers. The study population consisted of similar equal numbers of male and female infants, with ninety-eight males (49·0 %) and 102 females (51·0 %). The mean birth weight was 3·3 ± 0·4 kg, and the mean birth length was 51·1 ± 1·4 cm. The median APGAR scores at 1 min, 5 min and 10 min were 10·0, 10·0 and 10·0, respectively. These scores indicate that the infants were generally healthy at birth. Sixty-six participants (33·0 %) reported that they use supplements (including vitamin complex, vitamin D, vitamin C, DHA, Ca, Runkang brand pregnancy supplements and Forceval brand pregnancy supplements), but only seven of them used supplements containing iodine. The daily iodine dose in these supplements (n 7) amounted to 150 µg. No infants had a birth weight < 2500 g (i.e. low birth weight).
Table 1. Socio-demographic characteristics of women and their infants

SME, small and medium-size enterprise; IQR, interquartile range.
* Data are means ± sd, median (IQR) or n (%).
Iodine status
The iodine status of women is presented in Table 2. The overall median (interquartile range (IQR)) BMIC was 89 μg/l (74, 117 μg/l). The overall median UIC during pregnancy was 112 μg/l (85, 134 μg/l), which was indicative of iodine deficiency (median UIC < 150 μg/l), while the overall median UIC during lactation was 113 μg/l (90, 133 μg/l), indicating iodine sufficiency (median UIC ≥ 100 μg/l). No significant (correlations) differences were found between UIC during pregnancy and lactation (P = 0·784). The prevalence of iodine deficiency (as assessed by UIC) was significantly during pregnancy than that of lactation (69·8 % v. 30·2 %) (P < 0·001). The overall mean dietary iodine intake calculated from FFQ for pregnancy and lactation was 231·89 ± 146·02 and 237·26 ± 156·20 µg/d, respectively.
Table 2. Iodine status of women (n 200)

BMIC, breast milk iodine concentration; IQR, interquartile range; UIC, urinary iodine concentration; TSH, thyroid-stimulating hormone
* Data are means ± sd or n (%) or median and interquartile ranges (IQR). A P < 0·05 was taken as level of significance. P values are shown for comparison between pregnancy and lactation.
For those women who took iodine-containing supplements from pregnancy to lactation (n 7), all median UIC were below their respective median cut-off values: median UIC during pregnancy was 123 µg/l, and median UIC during lactation was 99 µg/l (median cut-off value 100 µg/l). Besides, the median BMIC of women who took iodine-containing supplements (n 7) was 97 µg/l.
Table 3 shows BMIC by socio-demographic features of women. There was no difference in BMIC in terms of different categories of age, BMI, delivery type, education, occupation and smoking status. We observed a positive significant correlation (r = 0·369, P < 0·001) between BMIC and UIC in breast-feeding women (Fig. 1).
Table 3. BMIC by socio-demographic characteristics of women

BMIC, breast milk iodine concentration.
* Data are median and interquartile ranges (IQR).
† Only three smokers.

Fig. 1. Scatter plots of 200 samples illustrating the correlation between BMIC and UIC during lactation. BMIC, breast milk iodine concentration; UIC, urinary iodine concentration
Usefulness of breast milk iodine concentration in the assessment of iodine status
Figure 2 shows the area under the receiver operating curve for BMIC using UIC as a reference standard was 0·755 (95 % CI: 0·644, 0·866), which was within the acceptable range (≥ 0·7)(Reference Hosmer, Lemeshow and Sturdivant29). This suggested a 75·5 % chance that BMIC would correctly distinguish iodine-deficient breast-feeding women from iodine sufficient breast-feeding women. Therefore, BMIC could be used as a biomarker of iodine status in breast-feeding women.
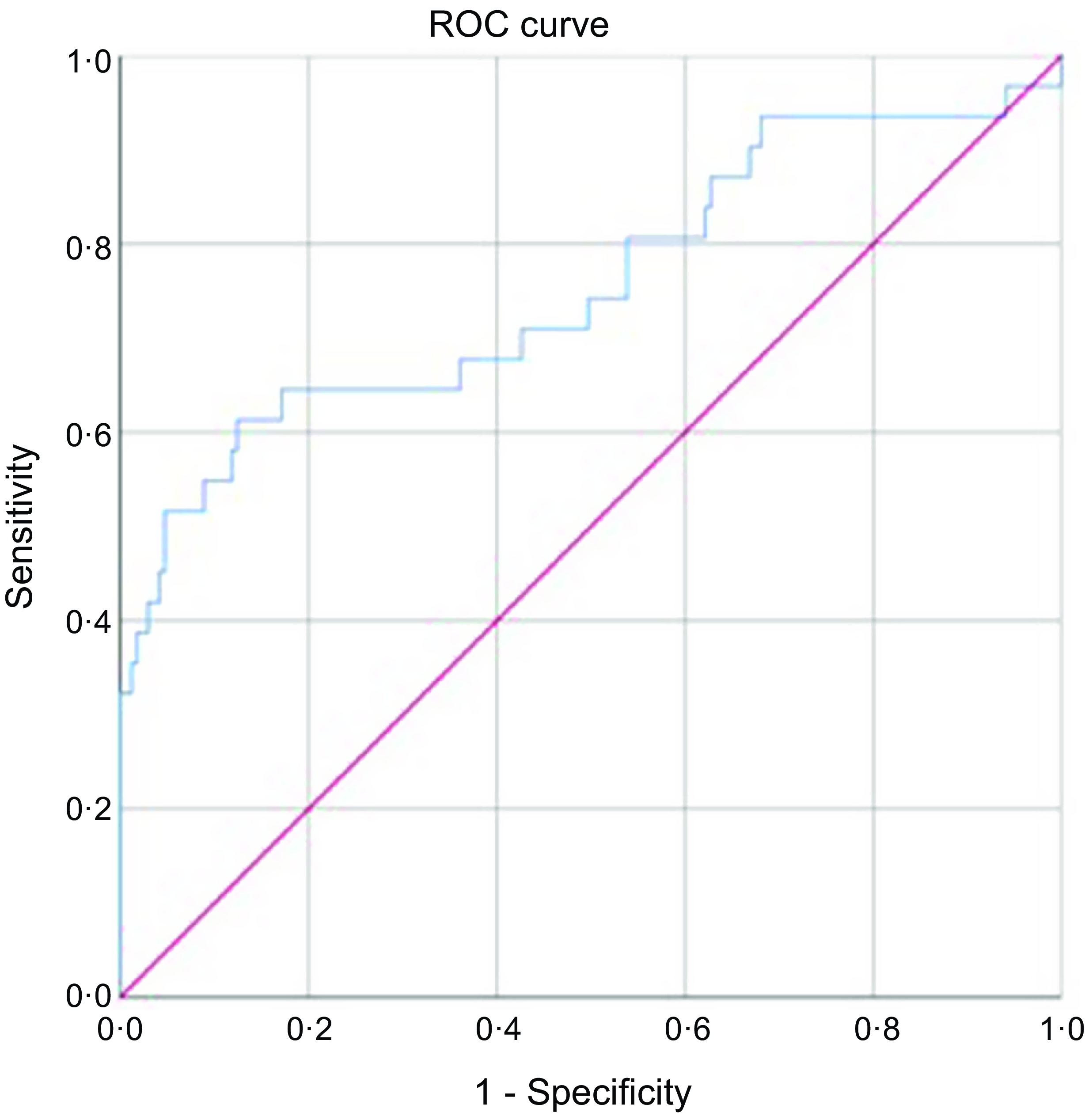
Fig. 2. The ROC curve for BMIC using UIC as a reference standard, diagonal line indicates chance (area = 0·5). ROC, receiver operating curve; BMIC, breast milk iodine concentration; UIC, urinary iodine concentration
Figure 3 indicates the optimal cut-off, in terms of optimising sensitivity and specificity of BMIC is 117 µg/l, with a sensitivity of 0·645 and a specificity of 0·828. This meant that if the cut-off value was used to test for iodine deficiency, it would correctly identify 64·5 % of participants who were iodine deficient and correctly identified 82·8 % of participants who were not iodine deficient. A negative predictive value of 92·6 % meant that 92·6 % of participants who tested negative for iodine deficiency actually did not have the deficiency, while a positive predictive value of 39·2 % meant that 39·2 % of participants who tested positive for iodine deficiency actually had the deficiency. These values indicated that the BMIC cut-off value of 117 µg/l was a good balance between accurately identifying iodine deficiency and avoiding false positives.

Fig. 3. Plots of sensitivity and specificity where lines cross are optimum value for classifying women as either being iodine sufficient or insufficient.
Table 4 shows the regression analysis for the predictors of BMIC in breast-feeding women. The only significant predictor of BMIC was UIC during pregnancy. This suggested that if a woman had a UIC pregnancy level greater than the UIC cut-off value, the woman was eight times more likely to have a BMIC above the optimal cut-off (i.e. 117 µg/l) when compared with a woman with a UIC pregnancy level below cut-off. However, the 95 % confidence level suggested that this difference may be as low as 3·5 times or as high as 18·6 times.
Table 4. Predictors of BMIC in women

BMIC, breast milk iodine concentration; UIC, urinary iodine concentration.
* Significant (P < 0·05).
† Adjusted for variables of age, UIC pregnancy, UIC lactation, delivery type, occupation and education in the model. Dependent variable: BMIC (cut-off of 117 µg/l).
‡ Categories for delivery type: 0 = natural birth, 1 = cesarean birth.
§ Categories for occupation: 0 = employed, 1 = non-employed.
|| Categories for education: 0 = completed high school or lower education, 1 = higher education.
¶ Categories for BMI: 0 = normal weight, 1 = under/overweight.
** Categories for use of iodised salt: 0 = use, 1 = not use/unknown.
†† Categories for use of supplement in pregnancy: 0 = use, 1 = not use.
‡‡ Categories for use of supplement in lactation: 0 = use, 1 = not use
Discussion
Our study was one of the first studies to use the BMIC reference range proposed by Dold et al., which is for exclusively breast-feeding women residing in iodine sufficient regions(Reference Dold, Zimmermann and Aboussad10). In our study, a number of biomarkers of iodine status including BMIC and UIC were employed to provide a comprehensive assessment of iodine status for women during their first week of lactation in an iodine sufficient region of China. This is because during lactation, about 45 % of maternal iodine is redirected to meet the infant’s iodine requirement, resulting in a decrease in fractional iodine excretion in the maternal urine(Reference Laurberg and Andersen33).
Currently, there is no consensus on an acceptable BMIC cut-off to categorise iodine sufficiency during lactation, which is primarily due to the uncertainty about infant iodine requirements. Several BMIC cut-offs of 50, 75, 80, 92 and 100 μg/l have been proposed to ensure iodine sufficiency during lactation(Reference Liu, Sharp and Villanueva11). However, these proposed BMIC cut-offs did not specify if they could be applied to breast-feeding women residing in iodine sufficient regions because some of these cut-offs may have been derived from iodine deficient breast-feeding women and therefore not suitable for iodine sufficient regions. For example, higher BMIC was reported in goitrous areas of Detroit than non-goitrous areas of Boston(Reference Turner34). Furthermore, no difference in BMIC was reported between goitrous and non-goitrous areas of Italy and New Zealand(Reference Hercus and Roberts35,Reference Vermiglio, Presti and Finocchiaro36) . On the other hand, in a large multi-centre study of lactating women, Dold et al. proposed a broad reference range of 60–465 μg/l to suggest iodine sufficiency in exclusively breast-feeding women from iodine sufficient regions(Reference Dold, Zimmermann and Aboussad10).
The overall median BMIC in the first week of lactation was 89 μg/l, which was indicative of iodine sufficiency based on the BMIC reference of 60–465 μg/l suggested by Dold et al.(Reference Dold, Zimmermann and Aboussad10). In addition, our overall median BMIC in the first week of lactation was within the range of BMIC (from 43 to 138 μg/l) in the first week of lactation as reported in previous studies(Reference Vermiglio, Presti and Finocchiaro36–Reference Kurtoglu, Akcakus and Kocaoglu42). Of these previous studies (n 7), only two of them were conducted in iodine sufficient lactating women(Reference Böhles, Aschenbrenner and Roth37,Reference Kart, Türkmen and Anık40) . Our overall median BMIC was higher than that of Böhles et al.(Reference Böhles, Aschenbrenner and Roth37) (55 μg/l), but lower than that of Kart et al.(Reference Kart, Türkmen and Anık40)(138 μg/l). This highlighted that there have been very few studies that have investigated BMIC in the first week of lactation and data particularly from iodine sufficient regions are limited.
WHO recommends that breastfed infants receive adequate amounts of iodine in their diet to ensure a normal growth and development. Breast milk is the only dietary source of iodine for breastfed infants. BMIC is primarily influenced by the maternal dietary iodine intake(Reference Kirk, Kroll and Dyke43). Women who had low iodine intake were reported to have a lower BMIC compared with women with sufficient iodine intake(Reference Liu, Sharp and Villanueva11). Other factors such as stages of lactation and the geographical location of the women have been reported to affect BMIC. Women who lived in regions with naturally low soil iodine concentration were likely to have lower BMIC(Reference Zimmermann44). Due to the collection of breast milk on the 3rd day postpartum, it is possible that some women would still be producing colostrum, while others are likely to be producing transitional milk(Reference Tudehope45). In studies from the USA, Germany, Italy, China, New Zealand, Korea and Morocco, BMIC appeared to be highest in colostrum and decreasing throughout lactation, although not all studies have reported this pattern(Reference Andersson and Braegger1,Reference Mulrine, Skeaff and Ferguson20,Reference Dror and Allen46–Reference Zhang, Zhao and Shan48) . Therefore, it is assumed that BMIC will fall below the reference range for later mature milk with the prolongation of lactation stages.
This study reported that the area under the receiver operating curve for BMIC using UIC as a reference standard was 0·755, which was within the acceptable range. In addition, using the plot of sensitivity and specificity, our study reported an optimal BMIC cut-off of 117 µg/l might be used for categorising iodine sufficiency in lactating women residing in an iodine sufficient region. Although there have been many proposed BMIC cut-offs to determine iodine sufficiency in lactating women, the most commonly used BMIC cut-offs in the literature are 75 and 100 μg/l(Reference Dold, Zimmermann and Aboussad10,Reference Semba and Delange22,Reference Bazrafshan, Mohammadian and Ordookhani49–Reference Delange51) . However, there is still no scientific consensus on the agreed BMIC cut-off. One of the possible reasons is because the iodine needs for infants are still inconclusive(Reference Andersson and Braegger1). In addition, the breastmilk samples from the published studies had different collection periods of lactation (varied from days/weeks to months), making the comparison of BMIC between studies with different breast milk collection periods of lactation difficult. More studies assessing BMIC along with UIC and thyroid function in mother–infant pairs are warranted to define a median BMIC cut-off for assessing iodine sufficiency in lactating women residing in iodine deficient and sufficient regions.
This study found that while women were iodine deficient (median UIC < 150 μg/l) during pregnancy, they were categorised as iodine sufficient (median UIC ≥ 100 μg/l) during lactation. Despite both the median UIC values of women during pregnancy and lactation were being similar (i.e. 112 μg/l and 113 μg/l, respectively), the categorisation of iodine status for pregnant and lactating women was different in this study. This suggested a change in the iodine profile of women through pregnancy and into lactation. The median UIC cut-off to determine iodine sufficiency in lactating women is 100 µg/l, which is lower than pregnant women because in lactating women, ingested iodine is excreted both in urine and breast milk(5). Therefore, due to the variation in the partition of iodine between breast milk and urine, both BMIC and UIC are recommended to be included when assessing iodine status in breast-feeding women(Reference Brough4). On the other hand, the median UIC cut-off value for classifying iodine sufficiency during pregnancy is 150 μg/l, which was established on the basis of an average daily urine volume of 1·5 l(5). However, during pregnancy, there is an increase in the glomerular filtration rate, which leads to increased daily urine volume and subsequently lowers UIC, and this may overestimate the prevalence of iodine deficiency in pregnant women(5,Reference Andersson, De Benoist and Delange9) .
Although the median UIC remained similar throughout pregnancy and lactation, the median BMIC of breast-feeding women was within the BMIC reference range proposed by Dold et al., which indicated iodine sufficiency(Reference Dold, Zimmermann and Aboussad10). This could be due to various factors such as a change in maternal iodine metabolism and increased iodine absorption characterised by increased thyroid stimulation, which may have contributed to an increase in iodine availability for secretion into breastmilk(Reference Eltom, Eltom and Elnagar52). Another possible reason may be that the mammary gland has the ability to selectively accumulate and concentrate iodine, independent of iodine intake(Reference Azizi and Smyth50). Therefore, it can actively transport iodine from the maternal bloodstream into breastmilk, resulting in higher BMIC values, even if the UIC value remains constant throughout these critical stages. However, the increased iodine uptake of the mammary gland may take place at the expense of maternal iodine reserves if there is insufficient maternal iodine intake from the women’s diet(Reference Fu, Gao and Guo53).
There are several challenges involved during the collection of breast milk samples from women in the early stages of lactation(Reference Zhao, Ding and Arya54). New mothers, particularly first-time mothers, are unlikely to interrupt breast-feeding at the early stage of lactation because this is a critical period for the establishment of exclusive breast-feeding(Reference Zhao, Ding and Arya54,Reference Agostoni, Braegger and Decsi55) . Our study had 57·5 % of first-time mothers. Furthermore, breast milk is considered precious and beneficial for infants in Chinese culture. For these reasons, it can be difficult to recruit women to take part in this type of research. Robust data on iodine status, especially BMIC, are scarce(Reference Liu, Sharp and Villanueva11). One of the challenges of this study was collecting breast milk samples from breast-feeding women due to limited volumes of breast milk produced. During the first week of lactation, only small volumes of breast milk are produced (i.e. mean volume on the first 24 h after birth and third day of lactation is 37·1 (range 7·0–122·5) g and 408 (range 98·3–775) g)(Reference Saint, Smith and Hartmann56). Breast-feeding women encountered difficulties expressing enough milk. Our data should be interpreted with caution because spot samples of breast milk and urine were used to assess iodine status in our study. Future studies should consider collecting multiple breast milk and urine samples over 24 h and wider time range to calculate the daily iodine excretion from lactating women with different iodine intake levels. This will provide a more comprehensive assessment of iodine status in lactating women.
This study has several strengths. First, two biomarkers of iodine status, BMIC and UIC were used in assessing iodine status of breast-feeding women. The UIC values during lactation were derived from spot urine samples collected from two consecutive days. Although it is suggested that at least ten spot urine samples are needed to reliably assess individual iodine status, it was not feasible to obtain in this study. It is suggested that two spot urine samples provide a better estimate of iodine status compared with a single spot urine sample(Reference König, Andersson and Hotz57). The participants in this study consisted of a rather homogenous group of women with similar age and BMI ranges. Second, breast milk samples were collected during the first week of lactation, which few studies have performed. Third, the urine and breast milk samples were collected during the same time period, which can reflect better comparability of the iodine status. In addition, to our knowledge, this was the first study to evaluate BMIC of breast-feeding women in China using the reference range of 60–465 μg/l proposed by Dold et al.(Reference Dold, Zimmermann and Aboussad10). The BMIC reported in our study was at the lower end of the reference range, which was likely influenced by the UIC of breast-feeding women, which was just above the UIC cut-off indicating iodine sufficiency. However, it is important to note that the reference range proposed by Dold et al. has a broad range and might not be applicable to breast-feeding women living in iodine deficient areas(Reference Dold, Zimmermann and Aboussad10). Also, the reference proposed by Dold et al. does not identify the BMIC value that corresponds to severe, moderate, mild iodine deficiency, optimal, more-than-adequate and excessive iodine status in breast-feeding women in the early, mid and later stages of lactation(Reference Dold, Zimmermann and Aboussad10).
Despite its considerable strengths, this study was limited by the failure to follow-up for a longer period of time to obtain subsequent samples. This is because the pandemic of coronavirus disease 2019 (COVID-19) affected some aspects of this study, including recruitment of participants because of the city lockdown, travel restrictions and hospital guidelines to prevent the spread of the COVID-19 pandemic. In addition, women usually left the hospital and return home on the 4th day of postpartum, making continued follow-up and sample collection difficult.
Conclusions
This study demonstrated that women were iodine sufficient in the first week of lactation. In addition, these findings supported the proposed BMIC reference range of 60–465 μg/l for a group of exclusively breast-feeding women in our region. Given the lack of conclusive evidence, more studies on the usefulness of BMIC as a biomarker of iodine status are warranted in breast-feeding women and infants with varying iodine status and lactation stages. Only then can reasonable recommendations be made regarding the usefulness of BMIC to assess their iodine status.
Acknowledgements
We were extremely grateful to all the participants who took part in the cohort study. We would also like to thank Sheila Skeaff for providing the intellectual input to the study.
This work was supported by the Research Development Fund (RDF) (reference no. RDF-18–01–15) from Xi’an Jiaotong-Liverpool University.
S. L. (Shuchang Liu), A. S, X. L., E. V. and Z. F. M. were responsible for the study design. S. L. (Shuchang Liu) was responsible for research tool development, participant recruitment, field investigation, data collection, statistical analysis and wrote the first draft of the manuscript. S. L. (Steven Lane) assisted with the statistical analysis. A. S, X. L., E. V., Z. L. and Z. F. M. provided oversight and leadership responsibility for the research activity planning and execution, including the mentorship for PhD candidate S. L. (Shuchang Liu). All authors read and approved the final manuscript. All authors reviewed the manuscript.
The authors declare that they have no conflict of interest.
All procedures performed in the retrospective study involving human participants were in accordance with the ethical standards of Xi’an Jiaotong-Liverpool University Ethics Committee (reference no. 20–01–09) and Xianyang Central Hospital Affiliated to the Medical Department of Xi’an Jiaotong University (reference no. 20200009). The procedures were complied with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.












