The brain is an energetically expensive organ despite its small size (approximately 1·3–1·4 kg), accounting for approximately 20 % of resting energy expenditure(Reference Wang, Ying and Bosy-Westphal1). From the moment of conception and through the various developmental stages, an optimal intake of energy and nutrients is essential for normal brain formation and neurocognitive development(Reference Vaivada, Ahsan and Zaman2). Environmental and/or genetic factors can affect nutritional status, especially if occurring during the early stages of development, and can often lead to various degrees of neurocognitive impairment(Reference Nyaradi, Li and Hickling3). Although rare, examples of genetic disorders include Prader–Willi syndrome, phenylketonuria and inherited metabolic disorders(Reference Chelly, Khelfaoui and Francis4). Examples of environmental factors include deficiencies of minerals (i.e. I, Fe) and vitamins (i.e., folic acid, vitamin A)(Reference Prado and Dewey5). This is particularly common in developing countries and still represents a public health concern.
Similar to other physiological parameters such as bone mass(Reference Harvey, Dennison and Cooper6) and lung function(Reference Agusti and Faner7), the trajectory of neurocognitive function is typically represented by the shape of a Maxwell–Boltzmann distribution curve(Reference Cabeza, Albert and Belleville8). This defines a maximum peak that is typically reached during early adulthood followed by progressive decline in performance with ageing(Reference Salthouse9). Characterising the typical cognitive profile associated with a healthy ageing trajectory is fundamental for identifying key risk and protective factors and the development of intervention and risk reduction strategies. Indeed, factors influencing neurocognitive trajectories could be compared with vectorial forces shaping the direction and velocity of a given trajectory, which would represent the cumulative result of the forces applied by protective and risky factors at each time point during the life course of an individual. Fig. 1 provides a graphical model of this concept applied to a healthy (left graph) and abnormal (right graph) cognitive trajectory. The model illustrates the complex and dynamic interaction that may happen at any point in an individual’s lifetime, which could shape the direction and velocity of the cognitive trajectory. The net balance between protective and modifiable/non-modifiable risk factors would determine the ascent rate and peak of the cognitive potential of an individual in early life; the subsequent rate of decline would be the result of the net effect of the ageing process, non-modifiable and modifiable protective and risk factors. In the context of dementia risk, a greater downtrend of the trajectory would be given by a greater negative net balance leading to accelerated cognitive decline, cognitive impairment and, if protracted, development of dementia. The reversibility of the stages of cognitive impairment is a contentious area, but the general consensus is for interventions to follow the simple rule of thumb ‘the earlier, the better’ as irreversibility may be difficult, if not impossible, once the onset of clinical dementia occurs(Reference Livingston, Huntley and Sommerlad10).

Fig. 1. This graph has been created based on the ‘pendulum’ model of disease risk(Reference Langie, Lara and Mathers72). The graph expands the concept by adding a vectorial dimension to the non-modifiable and modifiable factors that can shape the trajectories of cognition across the life course of an individual. First, a description of the key elements of the graph is needed. Arrows indicate vectorial forces resulting from the cumulative influences of protective (green) and risky (blue) modifiable risk factors. Black arrows indicate influence of modifiable risk factors on life course cognitive trajectories. The size of the arrows indicates the cumulative magnitude of the effects on the factors on the cognitive trajectories. The direction of the arrows indicates the applied cumulative force applied by factors to the cognitive trajectories. In a health trajectory, cognitive function achieves the greatest individual potential during the early life and starts to gradually decline as the influence of the ageing process (black arrows) progressively increases in magnitude but maintaining an overall normal cognitive function and staying well above the range of cognitive impairment (coloured areas). Influence of risky modifiable factors (blue arrows) may also increase later in life due to, for example, reduced physical mobility and diet quality. The abnormal trajectory on the right describes one of the possibly multiple scenarios leading to an accelerated cognitive decline that an individual may present during the life course with achievement of a lower cognitive potential followed by an accelerated cognitive decline due to greater net negative forces derived from the balance of non-modifiable and modifiable risky factors and modifiable protective factors. The result is an accelerated trajectory crossing into cognitive impairment and increasing the risk of developing severe cognitive impairment (i.e. dementia) within the lifetime of an individual.
Adherence to a healthy diet and achievement and maintenance of an optimal nutritional status are vital strategies to improve brain and cognitive health as captured in the term ‘nutritional psychiatry’(Reference Adan, van der Beek and Buitelaar11,Reference Firth, Gangwisch and Borsini12) . Research in this area has greatly expanded in the last two decades(Reference Ekstrand, Scheers and Rasmussen13) with observational and interventional studies testing the influence of various nutrients (i.e. caffeine(Reference Nehlig14), polyphenols(Reference Vauzour15), PUFA(Reference Bentsen16), B vitamins(Reference Kennedy17), vitamin D(Reference Anjum, Jaffery and Fayyaz18), dietary nitrates(Reference Clifford, Babateen and Shannon19)) and dietary patterns (i.e. Mediterranean Diet (MedDiet)(Reference Klimova, Novotny and Schlegel20), Dietary Approach to Stop Hypertension (DASH) and MIND diet(Reference Kheirouri and Alizadeh21)) on brain health alone or as part of multimodal interventions (i.e. Finger trial(Reference Ngandu, Lehtisalo and Solomon22), Encore study(Reference Smith, Blumenthal and Babyak23)). While the evidence has been overall modest and conflicting on the protective effects of single nutrients, more convincing evidence has emerged from the investigation of holistic, nutritional approaches based on promoting a greater adherence to healthy dietary patterns(Reference Chen, Maguire and Brodaty24). Shannon et al.(Reference Shannon, Stephan and Granic25) demonstrated that a higher MedDiet adherence, defined by the Pyramid MedDiet score, was associated with better global cognition, memory and executive function in older (i.e. ≥ 60 years) UK adults recruited from the European Prospective Investigation into Cancer and Nutrition–Norfolk (EPIC-Norfolk). Further, the Predimed intervention trial showed that a MedDiet supplemented with olive oil or nuts was associated with improved composite measures of cognitive function after 4 years of follow-up in adults aged 55–80 years(Reference Valls-Pedret, Sala-Vila and Serra-Mir26).
While these studies certainly have great potential for public health prevention, the mechanistic insights provided are limited as effects are likely to be derived from the synergy of different nutritional factors.
A compound that is increased in healthy dietary patterns, as it is closely associated with fruit and vegetable intake, is dietary nitrate(Reference Hord, Tang and Bryan27). It is estimated at the population level, in Western countries, that dietary nitrate intake is approximately 110 mg/d(Reference Babateen, Fornelli and Donini28). A previous review(Reference Hord, Tang and Bryan27) estimated that the nitrate content of healthy dietary patterns, such as the MedDiet or DASH diet, could be 10-fold higher (approximately 1000–1200 mg/d) than the estimated average nitrate intake of Western populations (approximately 110 mg/d)(Reference Babateen, Fornelli and Donini28) and considerably higher than the level of nitrate intake currently recommended by the WHO (3·7 mg/kg of body weight (corresponding to approximately 280 mg/d for a person with a body weight of 70 kg))(Reference l’Hirondel29). The protective effects of higher levels of nitrate intake (approximately 400–800 mg/d) on cardiometabolic and neurocognitive health have been consistently reported in randomised trials(Reference Clifford, Babateen and Shannon19,Reference Siervo, Scialò and Shannon30) . Some studies have also suggested an interaction with ageing such that older individuals may need higher nitrate doses to elicit similar effects on vascular outcomes to those observed in younger groups(Reference Capper, Clifford and Taylor31,Reference Siervo, Lara and Jajja32) .
Dietary nitrate and brain health
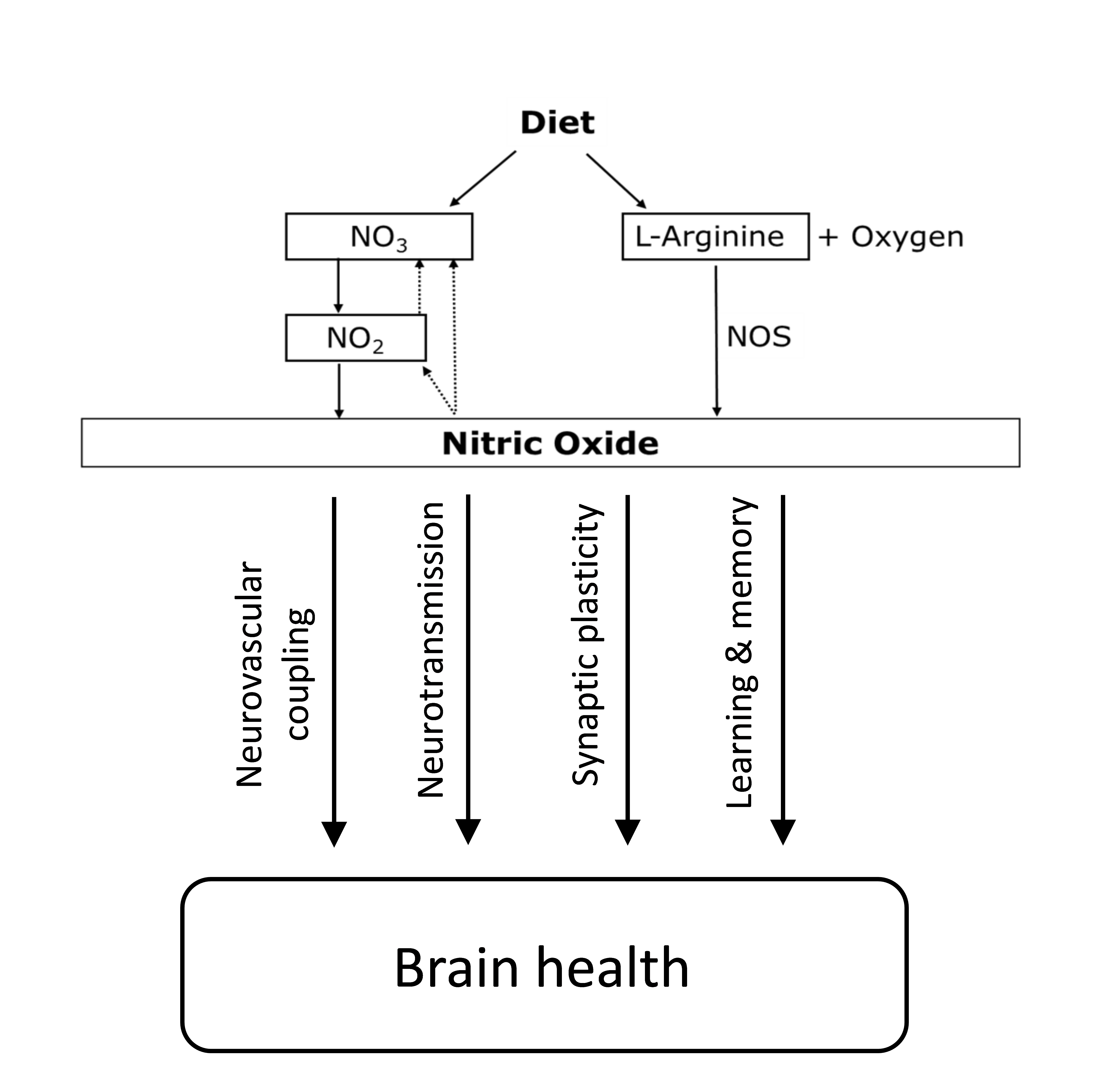
Inorganic nitrate is a water-soluble compound that can be found naturally in water and soil and is a fundamental component of the nitrogen cycle(Reference l’Hirondel29). Both nitrate and nitrite can be produced endogenously in humans via oxidation of nitric oxide (NO). Nitrate can be formed directly from the reaction between NO and oxy-Hb(Reference Gow, Luchsinger and Pawloski33), while nitrite can be produced through auto-oxidation of NO, which is catalysed by plasma protein ceruloplasmin(Reference Shiva, Wang and Ringwood34). Both are considered end products of endogenous NO metabolism(Reference Lundberg, Weitzberg and Gladwin35). In animal studies, NO can be formed under acidotic conditions by the reduction of the large pool of systemic nitrite, and this formation is not blocked even after NO synthase (NOS) inhibition(Reference Zweier, Wang and Samouilov36). These findings have also been observed in humans after the infusion of 75 mg of sodium nitrite into the forearms of healthy individuals, resulting in blood flow increasing by 175 %. Interestingly, similar to the animal studies, the generation of NO was not blocked after NOS inhibition by the infusion of NG-monomethyl-l-arginine (NOS inhibitor). Therefore, it appears that systemic nitrite represents a storage pool for NO generation(Reference Cosby, Partovi and Crawford37). It has also been reported that nitrate can be used as a substrate for systemic nitrite formation after observing a significant increase in plasma nitrate and nitrite following a nitrate load(Reference Lundberg and Govoni38). All aforementioned studies have suggested that nitrate and nitrite can be recycled physiologically in tissues to synthesise NO independently of the enzymatic NOS pathway and are heavily dependent on the entero-salivary circulation of the nitrate pathway(Reference Lundberg, Weitzberg and Gladwin35). This pathway offers a backup system to promote NO production when endogenous NO generation via the NOS pathway is impaired(Reference Carlström, Larsen and Nyström39).
NO is the biological effector of the putative protection that dietary nitrate exerts on brain function, and it has been found to be involved in learning and memory processes(Reference Paul and Ekambaram40). NO is a gaseous and highly reactive molecule that can diffuse quickly to surrounding tissues(Reference Picón-Pagès, Garcia-Buendia and Muñoz41). NO can be synthesised in neurons following the activation of N-methyl-d-aspartate receptors via the amino acid glutamate, and the first to observe this mechanism was Garthwaite et al.(Reference Garthwaite, Charles and Chess-Williams42). This activation leads to the influx of Ca++ into the nerve cell, thus activating NOS via Ca++/calmodulin binding, which ultimately generates NO(Reference Garry, Ezra and Rowland43). NOS is expressed in all brain cells, including vascular, neuronal and glial cells; thus, there is NO production in the brain(Reference Knott and Bossy-Wetzel44), which has been implicated in cerebrovascular regulation. One of the mechanisms that underlie the regulation of cerebral blood flow (CBF) is neurovascular coupling(Reference Garry, Ezra and Rowland43). There is also a growing body of evidence suggesting that NO plays substantial roles in various physiological processes, including the regulation of vascular resistance, neuromodulation and neurotransmission(Reference Tewari, Sah and Bawari45). The neurotransmitter action of NO is achieved by stimulating soluble guanylate cyclase and forming a second messenger molecule, cyclic guanosine monophosphate(Reference Susswein, Katzoff and Miller46). NO is also involved in the modulation of synaptic functions, and the enhancement of synaptic activity has been shown to be mediated by the activation of soluble guanylate cyclase(Reference Katusic and Austin47). The loss of eNOS-generated NO via the NOS inhibitor has been shown to be related to the up-regulation of amyloid precursor protein expression, and an increase in Aβ, demonstrating the importance of endothelial NO in modulating amyloid precursor protein within the brain(Reference Austin, Santhanam and Katusic48). The NO-cyclic guanosine monophosphate pathway could be an essential therapeutic target in preventing neurocognitive impairment(Reference Austin, Santhanam and Katusic48).
Dietary nitrate therefore has the mechanistic potential to influence brain functions; however, observational and clinical trials, overall, have contrasting results(Reference Clifford, Babateen and Shannon19,Reference Shannon, Easton and Shepherd49) . A meta-analysis conducted in 2018 exploring the effects of dietary nitrate supplementation on cognition and CBF(Reference Clifford, Babateen and Shannon19) found a lack of evidence for the benefits of dietary nitrate on both outcomes. The review also highlighted the limitations of the studies (small sample size, short duration and use of healthy populations), which could have contributed to the limited efficacy of the dietary nitrate interventions. Since the publication of the review, additional studies(Reference Babateen, Shannon and O’Brien50,Reference Vanhatalo, L’Heureux and Kelly51,Reference Fan, O’Donnell and Lanford52,Reference Woodward, Santos-Parker and Lubieniecki53,Reference Petrie, Rejeski and Basu54,Reference Justice, Johnson and DeVan55,Reference Gilchrist, Winyard and Fulford56) have been published on the topic; we have summarised in Fig. 2 a selection of studies that have investigated the effects of dietary nitrate on neurocognition and CBF following supplementation for at least 1 week and conducted in subjects at greater risk of cognitive impairment. Only two studies have concomitantly measured both neurocognition, CBF or brain metabolites(Reference Babateen, Shannon and O’Brien50,Reference Vanhatalo, L’Heureux and Kelly51) , which was measured by magnetic resonance spectroscopy(Reference Vanhatalo, L’Heureux and Kelly51) and near-infrared spectroscopy(Reference Babateen, Shannon and O’Brien50). Five studies(Reference Babateen, Shannon and O’Brien50,Reference Vanhatalo, L’Heureux and Kelly51,Reference Woodward, Santos-Parker and Lubieniecki53,Reference Justice, Johnson and DeVan55,Reference Gilchrist, Winyard and Fulford56) assessed changes in cognitive function, and three(Reference Vanhatalo, L’Heureux and Kelly51,Reference Justice, Johnson and DeVan55,Reference Gilchrist, Winyard and Fulford56) reported significant changes in executive function, vigilance and motor skills. Four studies measured CBF or changes in brain metabolites(Reference Babateen, Shannon and O’Brien50,Reference Vanhatalo, L’Heureux and Kelly51,Reference Fan, O’Donnell and Lanford52,Reference Petrie, Rejeski and Basu54) and the two studies reporting significant effects on CBF measured by MRI(Reference Petrie, Rejeski and Basu54) and near-infrared spectroscopy(Reference Fan, O’Donnell and Lanford52) were conducted in participants with cardiovascular conditions suggesting greater benefits of dietary nitrate supplementation in individuals with reduced NO production. Nevertheless, while some promising results have been reported, the evidence is still contrasting. This could be because of the short study duration (longest duration was 13 weeks(Reference Babateen, Shannon and O’Brien50)), small sample size (largest sample size was sixty-two participants(Reference Babateen, Shannon and O’Brien50)) and recruitment of healthy individuals with no evidence of cognitive impairment.

Fig. 2. GOfER diagram (Graphical Overview for Evidence Reviews) summarising main studies testing non-acute (duration of supplementation of at least 7 d) effects of dietary nitrate or nitrite on cognition and/or cerebral blood flow in humans. RCT, randomised clinical trial; P, parallel; CO, cross-over; BJ, beetroot juice; M, memory; E, executive function; MS, motor skills; G, global; VS, visuo-spatial; NRIS, near-infrared spectroscopy; CT, computerised tomography; PET, positron emission tomography; CBF, cerebral blood flow; TIA, transient ischaemic attack. The study by Vanhatalo et al. measured changes in brain metabolites using magnetic resonance spectroscopy.
Implications for research and future recommendations
Research into the potential applications of dietary nitrate as an aid to cognitive function is still in its infancy, and there is considerable scope for future investigation in this area. A schematic summary of the main priorities for future research is provided in Fig. 3. One approach which is starting to attract attention (see, e.g., Blekkenhorst et al.(Reference Blekkenhorst, Bondonno and Lewis57)), but could be further exploited, is the use of existing cohort studies to explore associations between nitrate intake with neurocognition and the risk of neurodegenerative diseases such as dementia. This relatively cost-effective approach could be applied to explore associations between dietary nitrate (including total nitrate intake and specific-nitrate-containing foods) and cognitive ageing in a real-world setting with longer follow-up durations and greater sample sizes than is typically feasibly in randomised clinical trials(Reference Black58). Such research may allow the identification of population sub-groups who may be particularly responsive to the effects of dietary nitrate and to identify potential effect moderators (e.g. genetic variants, age, sex, interactions with other dietary or lifestyle factors) which can then be used to inform the design of future randomised clinical trials(Reference Black58). While results should be interpreted with some caution – observational studies do not allow us to infer cause and effect and may be subject to issues such as reverse causality and residual confounding – findings could complement those obtained from more labour-intensive randomised clinical trials(Reference Faraoni and Schaefer59).

Fig. 3. Current evidence and proposal for a plan of action to conduct priority studies to advance knowledge on the effects of dietary nitrate (NO3) and nitrite (NO2) on brain health. NO, nitric oxide; BP, blood pressure; EF, endothelial function; CBF, cerebral blood flow.
Carefully designed randomised clinical trials are also needed to help better understand the efficacy of nitrate and mechanisms of action through which this polyatomic ion may influence neurocognitive function(Reference Clifford, Babateen and Shannon19). To date, most studies exploring the effects of nitrate on neurocognitive function are short in duration and use a small selection of cognitive tests which assess a limited set of cognitive domains (see Fig. 2). Larger trials with a longer duration of follow-up, ideally including multiple, comprehensive cognitive assessments over time to track cognitive trajectories, or assess hard clinical outcomes such as dementia incidence, would provide valuable insight. In this regard, it is possible that particularly demanding cognitive tasks are required to ‘tease out’ the potential benefits of nitrate on cognition. Future studies may wish to look at the potential additive or synergistic effects of administering nitrate as part of a combined intervention for improving cognitive ageing, whether alongside other dietary compounds which have shown promise in boosting cognition independently (e.g. n-3 fatty acids, sodium reduction(Reference Stevenson, Shannon and Minihane60)); dietary factors which may augment the effects of nitrate (e.g. polyphenols, vitamin C(Reference Ashor, Shannon and Werner61)) or parallel lifestyle interventions such as increased physical activity(Reference Xu, Wang and Wan62,Reference Sanders, Hortobágyi and la Bastide-van Gemert63) . Most current trials use healthy participants, and studies are warranted in different populations, such as those with a degree of cognitive impairment or poor cardiovascular health (for whom nitrate could potentially improve cognition via direct effects on the brain and indirect effects via the improved cardiovascular function(Reference Stephan, Harrison and Keage64)), and individuals with low baseline NO status (e.g. older and obese individuals(Reference Ashor, Chowdhury and Oggioni65)). Such cohorts may be more responsive to the potentially beneficial effects of nitrate on cognition. Female participants are underrepresented in the nitrate literature, and future studies should seek to understand the effects of this polyatomic ion on cognition in both sexes rather than assuming similar responses in males and females(Reference Wickham and Spriet66).
Future studies may wish to exploit further use of novel imaging techniques (e.g. MRI, PET, near-infrared spectroscopy) to better understand the effects of nitrate on brain volume and function. Use of new ‘omics’ approaches (e.g. genomics, metabolomics, transcriptomics, proteomics), which provide insight into the cellular processes underpinning diet-related responses, could also provide valuable mechanistic insight(Reference Sancesario and Bernardini67), and so too could the measurement of biomarkers of neurodegenerative diseases such as β-amyloid deposition following prolonged nitrate supplementation. Animal model investigations have previously been used to explore physiological mechanisms of nitrate, particularly at the vascular and muscle levels(Reference Ferguson, Hirai and Copp68,Reference Ferguson, Hirai and Copp69) , and may provide an opportunity to explicate brain-related changes occurring with nitrate supplementation. Nevertheless, results from animal studies of neurodegeneration should be treated with caution, as they do not fully account for the complexities of dementia in humans(Reference Balez and Ooi70). Clearly, there is much work to do in this promising research area, and time will tell if consuming nitrate to improve cognition really is a ‘NO brainer’.
Conclusions
In 2016, the NIH workshop on dietary nitrate(Reference Ahluwalia, Gladwin and Coleman71) advocated for more epidemiological research and more robust randomised trials to better define the predictive role of dietary nitrate consumption in the prevention and treatment of chronic diseases. However, the impact on cognitive function and dementia risk was missing. The current evidence points towards the potential, protective role of dietary nitrate on brain health. However, the available evidence is limited. Most importantly, there are no data from large prospective studies on the association of dietary nitrate intake with cognitive impairment or dementia risk. Further, there is a lack of large and prolonged randomised trials conducted in subjects with or at risk of cognitive impairment. These studies are urgently needed, and for now, it is ‘too much ado about nothing’ as there is still limited evidence.
Acknowledgements
None
During the writing of this article, O. M. S. was supported by the NuBrain consortium, which is funded by the UK Nutrition Research Partnership (UK NRP), an initiative supported by the Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council (BBSRC) and the National Institute for Health Research (NIHR) (MR/T001852/1).
The structure of the review was conceived by M. S. M. S., A. B. and O. S. drafted the manuscript, with M. S. taking a lead role. All authors critically revised the manuscript and approved the final version prior to submission.
The authors have no conflicts of interest to declare.