The mechanistic target of rapamycin (mTOR) pathway plays a crucial role in many human diseases, including cancer, diabetes and immune disorders. Intriguingly, these diseases are associated with aberrant hyperactivity of the mTOR pathway, which makes inhibitors of mTOR potentially effective therapeutics. One of the most exciting developments in recent years is the discovery that mTOR is a key regulator of lifespan in eukaryotes and contributes significantly to multiple age-related diseases (Ref. Reference Tsang1). These observations emphasise the importance of identifying and understanding the functional components of the mTOR signalling network, and recent studies have provided significant insights into the molecular architecture of the mTOR pathway (Refs Reference Sabatini2, Reference Zoncu, Efeyan and Sabatini3). Simultaneously, there has been a growing focus on the compound resveratrol (RSV), which is reported to have antioxidant and anti-inflammatory effects, anti-cancer effects and beneficial effects on metabolism. The aim of present paper is to review the emerging evidence for effects of RSV on the mTOR pathway. RSV modulates a number of specific proteins in the mTOR pathway and these interactions may have important implications for human health.
Resveratrol
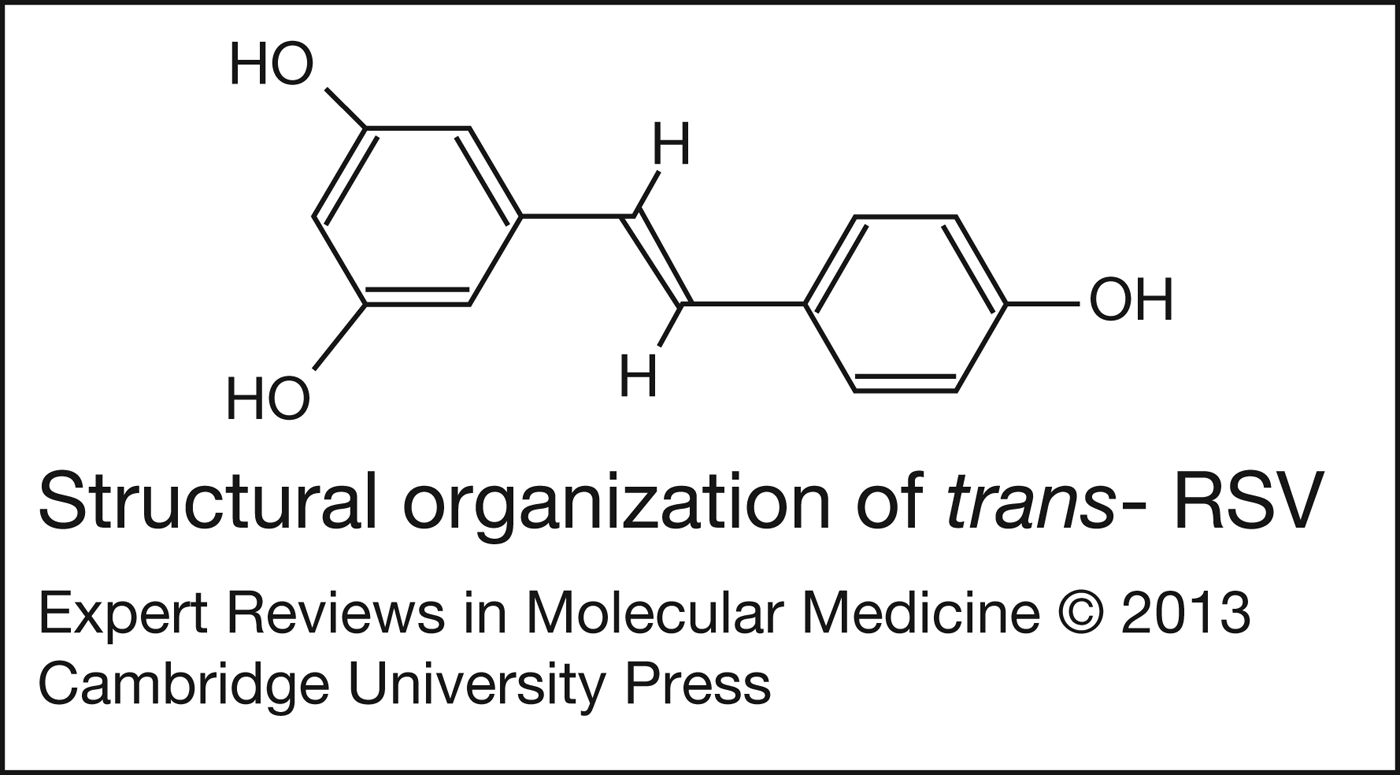
RSV (3,4′,5-trihydroxy-trans-stilbene) is a naturally occurring polyphenol (Fig. 1). RSV is found in low concentrations in more than 70 plant species including grapes, cranberries and peanuts, and also in a number of herbal remedies (Ref. Reference Shishodia and Aggarwal4). RSV from grapes is efficiently extracted during the process of winemaking, and it has been speculated that red wine, in particular, may be the most important dietary source of RSV. Notably, the RSV doses available in supplements and used in many clinical trials are 2–3 orders of magnitude beyond what could be obtained from the diet (Ref. Reference Vang5).

Figure 1. Structural organisation of trans-resveratrol (RSV).
Many of the health benefits of RSV are proposed to be mediated at least partly by suppression of low-grade inflammation, which is important for prevention of cancer, coronary heart diseases, diabetes and neurodegeneration (Refs Reference Sun6, Reference Sonnett7, Reference Speakman and Mitchell8). Similarly, variations in the cellular redox status are closely related to several diseases and are often found linked to the anti-inflammatory effect of RSV. Multiple biochemical and molecular actions, including changes in cell proliferation, apoptosis and angiogenesis, as well as suppression of DNA damage and inhibition of factors specific to metastasis are all proposed to contribute to RSV's effects against precancerous or cancer cells (Refs Reference Savouret and Quesne9, Reference Bishayee10). The alteration of xenobiotic metabolism by RSV probably plays a significant role in its cancer-preventive effect, but may also have an impact on drug metabolism. The induction of mitochondrial activity by RSV appears to be crucial in obesity/diabetes, but may also be important in its effects on longevity and influence Ca2+ uptake and signalling (Ref. Reference Agarwal and Baur11). Suppression of adipogenesis and stimulation of adipocyte lipolysis by RSV are significant when effect on obesity and diabetes are considered (Ref. Reference Szkudelska and Szkudelski12). Neuroprotection by RSV is likely caused by modulation of glutamate metabolism (Ref. Reference Quincozes-Santos13). RSV also stimulates osteogenesis and may stimulate bone formation or slow the progression of osteoporosis (Refs Reference Pearson14, Reference Mobasheri and Shakibaei15). During the last decade, RSV has been revealed to possess a fascinating spectrum of pharmacologic properties which could be useful in human medicine (Refs Reference Vang5, Reference Smoliga, Vang and Baur16).
Multiple human clinical trials have been completed with RSV, and are summarised in Vang in 2013 (Ref. Reference Vang17). However, the majority of experimental data that are available is from studies employing various animal models or human cells. Most of the human trials have been designed to evaluate the therapeutic effect of RSV rather than the disease-preventing effect, and most have involved a very short duration of treatment (Refs Reference Vang17, Reference Gescher, Steward and Brown18). Therefore, further studies in animal models similar to humans as well as in humans are needed in order to evaluate the chronic effect of RSV and to verify the lack of adverse effects in humans (Refs Reference Vang5, Reference Smoliga, Vang and Baur16).
It is important to note that often there are discrepancies between the doses of RSV used in cells, and the levels that are obtained in vivo. For example, many studies with RSV that show an impact on mTOR signalling are performed at concentrations in the range of 10–100 µM (Ref. Reference Scott19). In contrast, a single 25 mg dose of RSV, corresponding to high red wine consumption, resulted in marginal levels of plasma RSV in human subjects, and a 5 g bolus dose produced a transient peak of only 2.4 µM (Ref. Reference Boocock20). When lower concentrations corresponding to plasma levels are used on cells, outcomes are variable, and often no effect is detected (Ref. Reference Vang17). Leontieva et al. show no inhibition of mTOR activity by RSV at physiological levels in an in vitro experiment (Ref. Reference Leontieva21), which should be taken into account when interpreting results obtained using higher concentrations. RSV is well absorbed in the human gastrointestinal tract, although bioavailability of RSV in the human body is very limited because of rapid metabolism. In a separate study using lower daily doses of RSV, Almeida et al. (Ref. Reference Almeida22) showed that repeated dosing can increase the plasma half-life of RSV by more than twofold. In rabbits, rats and mice, tissue levels of RSV following oral delivery are closely parallel to plasma levels (Ref. Reference Asensi23), although much higher concentrations have been observed in human intestine following oral delivery (Ref. Reference Scott24). In considering these data, it should be noted that in virtually all in vitro experiments RSV concentrations are typically 10–100 µM and the duration of treatment is almost always less than one week, whereas in vivo studies may not achieve equally high concentrations in plasma, but the duration of exposure is often much longer. One should also bear in mind that RSV most likely has multiple physiologically relevant targets, many of which might be affected in different compartments at different concentrations.
Mechanistic target of rapamycin (mTOR)
mTOR is an evolutionarily conserved serine/threonine kinase that integrates signals from growth factors, nutrients and stress factors and controls multiple downstream processes, including mRNA translation, lipid and nucleotide synthesis, cell-cycle progression, autophagy and the shape and survival of cells (Ref. Reference Sarbassov, Ali and Sabatini25). The mTOR signalling pathway lies at the nexus of the regulatory network controlling anabolic processes. It represents one of the major growth and survival pathways that is dysregulated in many human cancers and contributes to cancer pathogenesis and therapy resistance (Refs Reference Pearson14, Reference Guertin and Sabatini26). On the other hand, the lack of mTOR signalling leads to autophagy, a catabolic process involving the degradation of the cell's own components through the lysosomal machinery. Therefore, rapamycin and other mTOR inhibitors (e.g. curcumin) are candidate anticancer drugs. These agents inhibit growth of a broad spectrum of cancer cells via mTOR suppression and can cooperate with other agents to induce apoptosis (Ref. Reference Petroulakis27). The structural organisation of mTOR protein is shown in Figure 3. mTOR serves as the catalytic subunit in two distinct protein kinase complexes, mTORC1 (mTOR Complex-1) and mTORC2 (mTOR Complex-2) (Ref. Reference Guertin and Sabatini26) (Fig. 2). mTORC1 has five core components: mTOR, the regulatory-associated protein of mTOR (Raptor), mammalian lethal with Sec13 protein 8 (mLST8, also known as GβL), proline-rich AKT substrate 40 kDa (PRAS40) and DEP-domain-containing mTOR-interacting protein (DEPTOR) (Refs Reference Peterson28, Reference Laplante and Sabatini29, Reference Laplante and Sabatini30). DEPTOR interacts with the mTOR via its structural PDZ domain (Ref. Reference Smoliga, Vang and Baur16). The core of mTORC2 comprises of six different proteins, several of which are common to mTORC1. mTORC2 contains mTOR, rapamycin-insensitive companion of mTOR (Rictor), mammalian stress-activated protein kinase interacting protein (mSIN1), protein observed with Rictor-1 (Protor-1), mLST8 and DEPTOR (Refs Reference Laplante and Sabatini29, Reference Abraham31). It has been demonstrated that mTOR is phosphorylated differentially when associated with mTORC1 or mTORC2, and that intact complexes are required for these phosphorylation events. Specifically, mTORC1 contains mTOR phosphorylated predominantly on S2448, whereas mTORC2 contains mTOR phosphorylated predominantly on S2481 (Ref. Reference Copp, Manning and Hunter32), although it should be noted that these marks are not completely exclusive (Ref. Reference Manning33). Under RSV treatment, mTOR phosphorylation of mTOR at serine 2448 was inhibited, whereas the phosphorylation of mTOR at serine 2481 was increased with low-dose RSV, but attenuated with high-dose RSV (Ref. Reference Leontieva21).

Figure 2. Structural domains of mechanistic target of rapamycin (mTOR). mTOR consists of: HEAT (Huntington-elongation factor 1A-protein phosphatase 2A-A subunit-TOR) repeats; a FAT (FRAP, ATM, TRRAP2) domain; the FRB (FKBP12-rapamycin-binding) domain, which is a conserved 11 kDa region necessary for FKBP12-rapamycin binding; a PIKK (PI 3-kinase-related kinase) domain; a regulatory domain (RD) and a FATC (FAT, C-terminal) domain. All of them are evolutionarily conserved in TOR orthologues. The amino acid residue number (top) shows the relative positions of the domains.
mTORC1 and mTORC2 have different targets and modes of regulation: mTORC1 is responsible for sensing nutrient signals and controlling cell growth (size) and proliferation in part by phosphorylating the downstream S6 serine/threonine kinase 1 (S6K1) and the eIF–4E-binding protein 1 (4E-BP1). mTORC2 is involved in the organisation of actin and thereby determining the shape of the cell. It also modulates cell survival in response to growth factors by phosphorylating its downstream effectors AKT, also known as protein kinase B (PKB), which plays a key role in multiple cellular processes such as glucose metabolism, cell proliferation, apoptosis, transcription and cell migration and serum/glucocorticoid regulated kinase 1 (SGK1) (Ref. Reference Guertin and Sabatini26).
Step-by-step description of the mTOR pathway
mTORC1 signalling regulates translation initiation by integrating several different inputs from nutrients including amino acids, as well as insulin and other hormones and growth factors activating the cell surface receptors, which activate the PI3 K/AKT signalling cascade (Fig. 3). mTORC2 serves as one of the kinases for AKT. Activated AKT phosphorylates TSC2 and destabilises the TSC1/2 complex (tuberous sclerosis complex), thus promoting the activation of mTORC1 because of the lack of inhibition of Rheb by TSC1/TSC2. Interestingly, this signalling to mTORC1 by AKT is largely intact in the absence of mTORC2, despite the fact that other AKT functions are disrupted (Ref. Reference Guertin40). On the other hand, Adenosine 5-monophosphate-activated protein kinase (AMPK) is modulated by the energy status (the AMP:ATP ratio) and upstream kinases including LKB1, and AMPK activates the TSC1/2 complex to repress mTORC1 signalling (Ref. Reference Sarbassov, Ali and Sabatini25). REDD1 protein (reregulated in development and DNA damage responses 1) is induced upon hypoxia, and also inhibits mTORC1 through activation of the TSC1/2 complex (not shown in Fig. 3). mTORC1 mediates the phosphorylation of 4E-BP1, S6K1 and eIF4G. Once activated, S6K1 phosphorylates ribosomal protein S6 and eIF4B. The net effect of these actions is an increase in translation.

Figure 3. Model for known players of the PI3 K-AKT-mTOR pathway that are modulated by resveratrol (RSV). Red boxes indicate inhibition and green boxes activation, respectively, by RSV. In relation to DEPTOR, RSV has an effect that results in decreased mTORC1 activity (Ref. Reference Liu34). Blue boxes indicate players that lack direct evidence for an effect of RSV. SIRT1: purple box, not a direct part of the mTOR pathway. RSV effects: inhibition of PI3 K/AKT/mTOR (Ref. Reference Zhou, Luo and Huang35); inhibition of mTORC1 via DEPTOR (Ref. Reference Liu34); inhibition of S6 phosphorylation (Ref. Reference Demidenko and Blagosklonny36); activation of AMPK (Refs Reference Dasgupta and Milbrandt37, Reference Vingtdeux38, Reference Kubota39); phosphatase and tensin homologue (PTEN), phosphatidylinositol 4,5-bisphosphate (PIP2), phosphatidylinositol-3-trisphosphate (PIP3), phosphatidylinositol-3-kinase (PI3 K), phosphoinositide dependent kinase 1 (PDK1), protein kinase B (AKT/PKB), tuberous sclerosis complex 1/2 (TSC1/TSC2), Ras homologue enriched in brain (Rheb), mTORC1; DEP-domain-containing mTOR-interacting protein (DEPTOR), regulatory-associated protein of mTOR (Raptor), mammalian lethal with Sec13 protein 8 (mLST8), eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), ribosomal protein S6 kinase (S6K1/2)(S6), mTORC2; rapamycin-insensitive companion of mTOR (Rictor), mammalian stress-activated protein kinas interacting protein (mSIN1), Activated Protein Kinase (AMPK), liver kinase B1 (LKB1), silent mating type information regulation 2 homologue 1 (SIRT1).
Phosphatidylinositol-3-kinase (PI3 K) is a lipid kinase, when activated, generates phosphatidylinositol-3,-4,-5-trisphosphate (PIP3). PIP3 is a second messenger essential for translocation of AKT to the plasma membrane. The PI3 K/AKT signalling pathway is activated by class IA PI3 K, wherein its regulatory subunits mediate activation of its p110 catalytic subunits by direct interaction with phosphorylated tyrosine residues of the activated receptors or adaptor proteins. AKT is phosphorylated and activated by phosphoinositide dependent kinase 1 (PDK1) (Fig. 3) and mTORC2 (Ref. Reference Sarbassov, Ali and Sabatini25) complex phosphorylates AKT on Ser473, which may facilitate PDK1-mediated phosphorylation of Thr308 (Ref. Reference Ravikumar41). Phosphatase and tensin homologue (PTEN) is a protein found in almost all tissues in the body that is encoded by a tumour suppressor gene. PTEN protein acts as a phosphatase to dephosphorylate PIP3, and specifically catalyses the dephosphorylation of the 3′-phosphate of the inositol ring in PIP3, resulting in the bi-phosphate product PIP2. This dephosphorylation results in inhibition of the AKT signalling pathway.
AMPK – adenosine 5-monophosphate-activated protein kinase
AMPK is a Ser/Thr protein kinase that exists as a heterotrimer in cells, composed of a catalytic kinase subunit (α) and two regulatory subunits (β and γ) (Ref. Reference Hardie42). The γ-subunit contains a series of cystathionine-β-synthase (CBS) domains that bind AMP (Ref. Reference Scott19), and binding AMP allows it to serve as a better substrate for upstream activating kinases such as LKB1 (Ref. Reference Kemp43). AMPK can also inhibit mTORC1 directly by phosphorylating the Raptor subunit (Ref. Reference Gwinn44). The kinase is activated in response to stresses that deplete cellular ATP and results in the formation of AMP, such as low glucose, hypoxia, ischaemia, heat shock and sepsis. AMPK is activated by phosphorylation within its activation loop at Thr172 (Refs Reference Hardie45, Reference Carling, Sanders and Woods46). The main AMPK-activating kinase is liver kinase B1 (LKB1), a protein expressed ubiquitously and recruited for AMPK phosphorylation after an elevation of the AMP:ATP ratio. LKB1 tumour suppressor is a serine/threonine kinase that is mutationally inactivated in the autosomal dominant Peutz–Jeghers syndrome (Ref. Reference Boudeau, Sapkota and Alessi47). The calcium/calmodulin-dependent protein kinase, kinase-β (CaMKKβ) is a second kinase that activates AMPK (not shown in Fig. 3), and others may exist. AMPK targets several proteins involved in cellular energy balance, including a key regulator of the switch between fatty acid biosynthesis and oxidation, acetyl-CoA carboxylase (ACC).
The AMPK pathway is linked to cell proliferation and tumour growth through the mTOR pathway. The calcium/CaMKKβ/AMPK signalling pathway controls mechanisms related to protein degradation through inhibition of mTOR signalling and the induction of autophagy (Ref. Reference Hoyer-Hansen48). Inflammatory stimuli induce the production of reactive oxygen species and increase intracellular calcium concentrations (Ref. Reference Hanisch and Kettenmann49), which can also contribute to the activation of AMPK-activating kinases (Ref. Reference Tamas50). Owing to its role as a central regulator of both lipid and glucose metabolism, AMPK is considered to be a key therapeutic target for treatment of obesity, type II diabetes mellitus and cancer. Overall, AMPK may serve as a critical modulator of ageing through its interactions with both mTORC1 and SIRT1, as well as its independent actions (Ref. Reference Shackelford and Shaw51).
4E-BP1 – eukaryotic translation initiation factor 4E-binding protein 1
Assembly of the eIF4F complex is inhibited by a family of repressor polypeptides, the eIF4E-binding proteins (4E-BPs) (Ref. Reference Gingras52). 4E-BP1 is a protein that, in humans, is encoded by the EIF4EBP1 gene. When 4E-BP1 is hypo-phosphorylated, it acts as a repressor of cap-dependent translation by binding to eukaryotic translation initiation factor 4E (eIF4E) and preventing its assembly into the eIF4F translation initiation complex at the 5′ end of mRNAs. The eIF4E subunit interacts directly with the mRNA 5′ cap structure in this complex. Following growth factor or mitogen stimulation, 4E-BP1 is sequentially phosphorylated on a number of sites by mTORC1, causing its dissociation from eIF4E and thereby promoting cap-dependent translation (Ref. Reference Sarbassov, Ali and Sabatini25).
S6K1-ribosomal protein S6 kinase
Mammalian cells contain two similar S6 kinase proteins, S6K1 and S6K2 (Refs Reference Reinhard, Thomas and Kozma53, Reference Martin and Blenis54). S6K2, which has 70% overall amino acid identity with S6K1, was discovered much later than S6K1 (Ref. Reference Shima55). Both isoforms, S6K1 and S6K2, are regulated by mTORC1 (Refs Reference Price56, Reference Park57), although most of the available data pertain to S6K1. S6K1, together with 4E-BP1, are two of the best characterised downstream effectors of mTORC1 (Fig. 3). S6K1 is phosphorylated directly by mTORC1 during stimulation with either nutrients or growth factors, and stimulates increased translation of mRNA through multiple downstream substrates (Refs Reference Tsang1, Reference Sarbassov, Ali and Sabatini25, Reference Holz58). S6K1 phosphorylates and activates the 40S ribosomal S6 protein, and is a direct downstream target of mTORC1. Huang et al. demonstrated that overexpression of SIRT1 was accompanied by enhanced activation of S6 K signalling and that rapamycin treatment of SIRT1 transfected cells reduced the phosphorylation of S6K1, implying a connection between SIRT1 and mTORC1 activity (Ref. Reference Huang59).
Modulation of mTOR cascade proteins by RSV
RSV's effect on specific proteins in the mTOR pathway
Many experiments have been carried out to define the effect of RSV on the mTOR pathway proteins and generally, the reported effects have been either down-regulated or no change (Fig. 4). Here, we present RSV's effects on the specific proteins in the mTOR pathway, including a very brief description of SIRT1's role in the regulation of the mTOR cascade. The inhibitory effects of RSV are also represented in Figure 3.

Figure 4. Overview of interaction of resveratrol (RSV) on proteins in the mTOR cascade. The numbers and the sizes of the bars refer to the number of articles which have investigated an effect of RSV on the specific protein. Bars indicate whether the protein was found to be up- or down-regulated (green and red bar respectively). No effect means that it has been tested but no change was detected (blue bar) (see supplemental table 1 for the full list of articles and details on the mechanisms used).
PI3 K/AKT inhibition by RSV
The inhibitory effects of RSV on the PI3 K/AKT signalling pathway and mTOR further down have been observed in multiple cell lines. Kueck et al. showed that RSV inhibited AKT phosphorylation in a dose- and time-dependent manner, which could provide a SIRT1-independent pathway to account for an observed decrease in glucose uptake (Ref. Reference Kueck60). This result further suggested that RSV would cause the inhibition of mTORC1 signalling downstream of AKT. Fröjdö et al. performed a similar series of experiments, but with another cell line (Ref. Reference Fröjdö61) and their results indicated that RSV affects both basal and insulin-stimulated glucose uptake, which has been supported in other cell types (Refs Reference Gingras52, Reference Fröjdö61, Reference Park62). In human U251 glioma cells, RSV down-regulates AKT and mTOR phosphorylation (Ref. Reference Jiang63). Further, RSV inhibited activation of the PI3 K/PDK1/AKT/pathway by oxidised LDL, and suppressed DNA synthesis and proliferation of cells were observed (Ref. Reference Brito64). Therefore, Zhou et al. classified RSV as an inhibitor of the PI3 K/AKT/mTOR signalling pathway (Ref. Reference Zhou, Luo and Huang35) (Figs 3 and 4). Also, Chen et al. found that RSV inhibited the phosphorylation of PI3 K, AKT and mTOR, and their results suggested that both PI3 K/AKT and mTORC1 play a major role in mediating anti-apoptotic effects of RSV (Ref. Reference Chen65). He et al. found that RSV suppressed the phosphorylation and activation of the PI3 K/AKT pathway in three breast cancer cell lines. Results indicate that RSV suppresses the phosphorylation of AKT (Ref. Reference He66). Fagone et al. sought to unravel the molecular mechanisms that underlie the inhibition of TGF-β-induced profibrotic effects by RSV in lung fibroblasts and found that TGF-β-induced phosphorylation of ERK1/2 and AKT was significantly inhibited by pretreatment with RSV (Ref. Reference Fagone67). Gurusamy et al. found that treatment with RSV inhibits mTORC1 phosphorylation dose dependently (Ref. Reference Gurusamy68).
Waite et al. investigated the ability of RSV to regulate PTEN protein levels in a breast cancer cell line. They observed that RSV increased PTEN protein levels and caused an apparent decrease in the level of phosphorylated AKT (Refs Reference Reinhard, Thomas and Kozma53, Reference Gurusamy68, Reference Waite, Sinden and Eng69). A combination of curcumin (a proposed mTOR inhibitor) and RSV effectively inhibited cell growth and induced apoptosis in in vitro studies. Murine PTEN-Cap8 prostate cancer cells showed a decreased cell growth when exposed to 10 µM RSV or curcumin but in combination (5 µM of each), the effect was significantly stronger than either RSV or curcumin alone. In PTEN-Cap8 cells, RSV was found to inhibit AKT and mTOR (Ref. Reference Narayanan70). This was supported by He et al., who showed that PTEN played an important role in RSV's growth suppressive effects and its potentiation of rapamycin (Ref. Reference He66). He et al. showed that RSV at a concentration of 10 µM for 48 h alone or in combination with different adenosine analogues caused a reduction of PTEN promoter methylation gene in MCF-7 breast cancer cells (Ref. Reference He66). This reduction of PTEN promoter methylation is associated with induction of the PTEN gene, as well as down-regulation of DNA methyltransferase and up-regulation of p21 (Refs Reference Shima55, Reference Narayanan70, Reference Stefanska71). Fagone et al. examined whether RSV alters PTEN expression levels, since PTEN expression levels and PTEN activity both are inversely correlated with AKT phosphorylation, alpha-actin-2 expression, cell proliferation and collagen production. They found that RSV inhibits the activation of PTEN in human lung fibroblast (Ref. Reference Fagone67).
AMPK-dependent effects
There is considerable debate about the mechanism by which RSV regulated SIRT1. Some show that a direct activation of SIRT1 by RSV is an in vitro artefact (Refs Reference Borra, Smith and Denu72, Reference Kaeberlein73, Reference Beher74, Reference Pacholec75) and that RSV works primarily by activating AMPK potentially by inhibition of phosphodiesterases (PDEs), ATPase or complex III (Refs Reference Zini76, Reference Gledhill77, Reference Hawley78, Reference Park79). Alternatively, RSV may first activate SIRT1 in vivo, leading to AMPK activation via deacetylation and activation of the AMPK kinase LKB1 (Refs Reference Hou80, Reference Ivanov81, Reference Lan82). mTORC1 is a potent repressor of autophagy and is negatively controlled by AMPK (Refs Reference Hardie45, Reference Carling, Sanders and Woods46). RSV promotes autophagy through AMPK-dependent inhibition of the mTOR pathway (Refs Reference Dasgupta and Milbrandt37, Reference Vingtdeux38). Puissant et al. verified that RSV is involved in autophagy in CML cell lines (Ref. Reference Fagone67). They found that RSV increased AMPK phosphorylation, which was accompanied by a net decrease in the status of phosphorylation of mTORC1, pS6 K, S6 ribosomal protein and the 4E-BP1, which could suggest a block at the TSC1/TSC2 complex (Ref. Reference Puissant83). Yi et al. showed that lipopolysaccharide (LPS) treatment significantly increased AMPK phosphorylation. However, pretreatment with RSV significantly inhibited LPS-induced AMPK phosphorylation (Ref. Reference Carling, Sanders and Woods46). Their findings indicate that RSV pretreatment provides a ‘preconditioning’ state for a decrease in the ATP:AMP ratio and then counteracts the LPS-activated AMPK phosphorylation in macrophage cells (Ref. Reference Yi84). Gurusamy et al. show that low doses of RSV extensively induce the activation of AMPK at Thr172 in H9c2 cells (Ref. Reference Gurusamy68). Hou et al. show that RSV activates AMPK in intact cells via an indirect mechanism in hepatocytes by activating SIRT1 and AMPK and that SIRT1 plays an essential role in LKB1/AMPK signalling in the regulation of hepatocyte lipid metabolism (Ref. Reference Hou80).
LKB1 activation (Ref. Reference Lin85)
Two upstream kinases have been identified as activators of AMPK, the tumour suppressor LKB1 and the CaMKKβ, which is not a direct part of the mTORC1 pathway (Refs Reference Hong86, Reference Hawley87). In fibrosarcoma cell line HT1080, LKB1 is shown to be the major upstream kinase of AMPK (Ref. Reference Shaw88). Chan et al. suggested that RSV exerts anti-hypertrophic effects by activating AMPK via LKB1 and inhibiting A, thus suppressing protein synthesis and gene transcription (Ref. Reference Chan89). Wt and LKB1-null MEFs cells were treated with 100 µM RSV for 1 h, which increased the phosphorylation status of AMPK in wt MEFs but not in the LKB1-null MEFs, suggesting that RSV effect on AMPK is mediated via LKB1. These findings are entirely consistent with what was observed in mouse neuroblast (Neuro2a) cells, where a significant activation of AMPK was found in RSV-treated cells simultaneously with no change in the AMP:ATP ratio (Refs Reference Dasgupta and Milbrandt37, Reference Chan89). Experiments by Dolinsky et al. indicated that RSV can prevent inhibition of the LKB1/AMPK energy-sensing pathway (Ref. Reference Dolinsky90). Treatment of cardiomyocytes with RSV prevents 4-hydroxy-2-nonenal (HNE)-induced modification of the LKB1/AMPK signalling axis and blunts prohypertrophic S6K1 kinase response. Consistent with inhibition of the LKB1/AMPK signalling pathway by HNE, the mTORC1/S6K1 kinase system is activated in the presence of 100 µM RSV, which is permissive for cardiac myocyte cell growth. Furthermore, administration of RSV to spontaneously hypertensive rats caused increased AMPK phosphorylation and activity and reduced left ventricular hypertrophy (Ref. Reference Dolinsky90). Treatment with RSV prevents acetylation of LKB1 and restores its activity in glomerular epithelial cells treated with high levels of glucose in a SIRT1 independent manner. Lee at al. showed that RSV regulates LKB1 activation in glomerular epithelial cells (Ref. Reference Lee91).
4EBP1 inhibition
There has been some interest in signalling via mTORC1 and previous works demonstrate that the metabolic actions of RSV require AMPK (Ref. Reference Dasgupta and Milbrandt37). Once activated, AMPK inhibited 4E-BP1 signalling and mRNA translation. The best understood role of mTORC1 in mammalian cells are related to the control of mRNA translation by 4E-BP1 (Refs Reference Gingras92, Reference Mothe-Satney93, Reference Wang94). In human breast cancer cell lines, RSV activated AMPK with decreased phosphorylation of mTORC1 and 4E-BP1. Lin et al. showed that RSV caused AMPK activation and a decrease of general mRNA translation in various cell lines, but these effects were not associated with significant effect on the total 4E-BP1 protein level (Ref. Reference Lin85). RSV does not have an inhibitory effect on 4EBP1 (Refs Reference Liu34, Reference Puissant83, Reference Lin85, Reference Lee91, Reference Puissant and Auberger95, Reference Villa-Cuesta96, Reference Tillu97).
S6K1 inhibition
A large-scale in vitro kinase screen identified S6K1 as a direct target of RSV, raising the possibility that the beneficial effects of RSV are because of modulation of S6K1 activity. In addition, it was shown that inhibition of S6K1 by RSV can prevent the full induction of autophagy in mammalian cells. This process is often used by cells to promote survivals under adverse conditions, for example, stress signals and nutrient deprivation. In contrast to the activation of the autophagy pathway observed in tumour cells in complete media, RSV markedly inhibits the starvation-induced autophagy response (Ref. Reference Morselli98). Armour et al. showed that this effect of RSV does not require SIRT1, and identified S6K1 as the target of RSV that is responsible for the inhibition of starvation-induced autophagy (Ref. Reference Armour99). Demidenko and Blagosklonny found that preservation of proliferative potential by RSV correlated with inhibition of S6K1 phosphorylation, and showed that RSV potently inhibited the phosphorylation of the S6K1 target, S6 (Refs Reference Ravikumar41, Reference Zhou, Luo and Huang35, Reference Demidenko and Blagosklonny36). Rajapakse et al. found that the increased activity of S6K1 in senescent compared to young Human Umbilical Vein Endothelial Cells (HUVEC) was inhibited by RSV (Ref. Reference Rajapakse100). Gurusamy et al. analysed the role of mTORC1 in relation to S6K1 which was highly active during hypoxia-reoxygenation, and showed that the activation of S6K1 was dose-dependently reduced by RSV treatment (Ref. Reference Leontieva21). Low doses of RSV extensively induced the activation of AMPK, compared with hypoxia-reoxygenation alone or higher doses of RSV (Ref. Reference Gurusamy68). Wei et al. tested the level of S6K1 in the mouse brain of Ppt1-KO mice fed on a diet with or without RSV and found the level significantly reduced (Ref. Reference Hou80). In the same experiment, they also found that PI3 K and AKT, which up-regulates mTORC1 activity by its ability to phosphorylate S6K1, was also reduced in the Ppt1-KO mice (Ref. Reference Wei101).
DEPTOR inhibition
DEPTOR is regarded as a negative regulator of the mTORC1 and mTORC2 signalling pathways and inhibits the kinase activity of both complexes. DEPTOR regulates protein synthesis and cell growth by inhibiting the ability of mTORC1 to phosphorylate downstream target proteins such as ribosomal S6K1 and 4E-BP1. DEPTOR is involved in regulation of apoptosis and the cell size (Refs Reference Sabatini2, Reference Peterson28). RSV was recently shown to increase the association between mTOR and DEPTOR, providing further evidence that DEPTOR could be involved in the RSV-induced inhibition of mTOR signalling (Ref. Reference Liu34). Data has not yet been presented to indicate direct binding of RSV to DEPTOR.
Inhibition of mTOR by RSV via SIRT1 dependent effects
RSV has been claimed to be an activator of SIRT1, a nicotinamide adenine dinucleotide (NAD+)–dependent deacetylase (Refs Reference Scott19, Reference Liu34, Reference Zhou, Luo and Huang35, Reference Demidenko and Blagosklonny36); however, this has been disputed (Refs Reference Dasgupta and Milbrandt37, Reference Vingtdeux38). Studies show that RSV increases the expression level of SIRT1, and might increase NAD availability via AMPK (Ref. Reference Park114) suggesting that it could increase the activity of SIRT1, though not necessarily by direct activation (Refs Reference Kubota39, Reference Reinhard, Thomas and Kozma53). Notably, a recent study has affirmed the conclusion that RSV and other small molecules can directly activate SIRT1 (Ref. Reference Hubbard102). Regardless of the mode of activation, SIRT1 mediates negative regulation of mTORC1 signalling through its association with TSC2—the prominent upstream inhibitor of mTORC1 signalling (Ref. Reference Shima55).
Lin et al. investigated whether RSV influences proliferation and protein translation via SIRT1 (Ref. Reference Gurusamy68), possibly via activation of the LKB1/AMPK pathway to suppress mTORC1 signalling (Ref. Reference Hou80). Lin et al. demonstrated in their study that RSV modulated translation and proliferation of oestrogen receptor-positive and oestrogen receptor-negative cells through AMPK activation that is dependent on SIRT1 (Ref. Reference Gurusamy68). Both MDA-MB231 and MCF-7 tumour cell lines were stimulated with 40 µM RSV. SIRT1 inhibitors attenuated the robust activation of AMPK by RSV for 72 h as judged by the increased phosphorylation of AMPK and reduced phosphorylation of its downstream effectors mTORC1 and 4E-BP1 (Ref. Reference Lin85).
Ghosh et al. also investigated the potential regulation of mTORC1 signalling by SIRT1 in response to nutrients and growth conditions (Ref. Reference Shima55). Cells were treated with 100 µM RSV and examined for phosphorylation levels of S6 and 4E-BP1. In mouse embryonic fibroblasts (MEF) and human HeLa cells, they found that the absence of SIRT1 resulted in higher phosphorylation of mTORC1, S6K1, 4E-BP1 and S6, suggesting that SIRT1 negatively regulates mTORC1. They concluded that RSV suppressed mTORC1 signalling via a negative regulation by SIRT1 (Ref. Reference Ghosh, McBurney and Robbins103). Ghosh et al. examined RSV's effect on mTORC1 activity in SIRT1 null cells and found that RSV treatment inhibited S6 phosphorylation in WT MEFs in a dose-dependent manner, but had reduced efficacy in SIRT1 null MEFs. This suggests that RSV's effect on mTORC1 signalling is mediated partly through TSC1/2 associated with SIRT1 (Ref. Reference Ghosh, McBurney and Robbins103).
Summary
Overall, the evidence presented herein supports the conclusion that RSV can inhibit the mTOR pathway. The mTOR cascade plays a crucial role in the determination of cell proliferation and growth, balancing the input of growth factors, energy and amino acids. Therefore, inhibition of mTOR has important implications for both ageing and cancer research, which is supported by the observation that this pathway is aberrantly activated in many human cancers (Refs Reference Ruggero104, Reference Wendel105). Thus, approaches to block the pathway are being actively pursued in many laboratories and pharmaceutical companies. Because mTOR is regulated by multiple factors, there are a number of target proteins for which intervention would be predicted to lower mTOR activity and have an impact on cancer, and possibly other age-related diseases.
Hahn-Windgassen et al. suggest that AKT/PKB can signal via mTORC1 by decreasing the AMP:ATP ratio and thus preventing AMPK from activating TSC1/TSC2 (Ref. Reference Hahn-Windgassen106). In addition, AKT/PKB regulates the influx of nutrients that activate the mTORC1 pathway (Ref. Reference Edinger and Thompson107). It has been suggested that the regulation of mTORC1-mediated fat metabolism involves signalling through S6K1 and 4E-BP1. More direct evidence of mTORC1–S6K1 pathway's role in fat metabolism has been obtained from the S6K1-mutant mice used by Um et al. (Ref. Reference Um108). These observations indicate that S6K1 and 4E-BP1 might be novel therapeutic targets for the development of anti-obesity drugs, and as presented in Figures 3 and 4, RSV inhibits S6K1/2 and 4EB-P1. Even though 4E-BP1 is one of the best characterised downstream effectors of mTORC1, data concerning its interactions with RSV are limited. Further studies are required to eliminate or confirm 4E-BP1 as a relevant target for RSV in vivo.
RSV stimulates AMPK activation likely by activation of the AMPK kinase LKB1; however, its activation is independent of SIRT1. Although the exact mechanism of RSV-mediated AMPK activation is unclear, there are four proposed mechanisms: inhibition of complex I, SIRT1-dependent LKB1 activation, SIRT1-independent LKB1 activation and PDE inhibition (Ref. Reference Park79). Because both SIRT1 and AMPK are involved in responses to metabolic stress, it will be interesting to determine whether there are additional interactions between these two protein families in neurons and other cells (Refs Reference Canto109, Reference Price110). These observations coupled with the demonstration that RSV can activate AMPK and lead to inhibition of ACC suggest that many calorie restriction mimetic actions of RSV might depend on the AMPK cascade. Indeed, the multiple beneficial effects of RSV may be because of its ability to alter the activity of multiple proteins involved in the cellular response to stress (i.e. SIRT1 and AMPK). The effect of RSV on AMPK is mediated via LKB1 and findings indicate that RSV can prevent inhibition of the LKB1/AMPK signalling pathway of mTORC1. Whether RSV has a direct effect on LKB1 or is mediated via another effector molecule is currently unknown (Ref. Reference Chan89). Several research groups have reported that LKB1 is a key upstream kinase for AMPK (Refs Reference Woods111, Reference Lizcano112, Reference Hardie113).
Evidence indicates that SIRT1 plays a role in the inhibition of mTORC1 signalling by RSV, but exactly where SIRT1 influences the pathway, and how relevant these mechanisms are in vivo, still need to be investigated. Experiments by Demidenko and Blagosklonny in 2009 showed that low concentrations of RSV do not inhibit S6 phosphorylation in culture. However, normal cells in the organism might be more sensitive to RSV. So, a crucial question is whether RSV can inhibit S6 phosphorylation in adipocytes, hepatocytes, muscle and other relevant cell types in the organism (Ref. Reference Demidenko and Blagosklonny36). DEPTOR is an additional protein in the mTOR complexes, which is regulated by RSV (Ref. Reference Liu34). The mechanism by which RSV promotes the binding of DEPTOR to mTOR complexes remains unknown, and could be because of direct binding or effects on upstream pathways. Therefore, there are likely to be multiple important mechanisms by which RSV negatively regulates mTOR signalling and function.
Perspectives
Over the last decade, knowledge of the mTOR signalling pathway has increased greatly, enabling researchers to better understand the mechanism of diseases such as cancer and type II diabetes. Despite these advances, our understanding of this signalling network is far from complete and many important questions remain to be answered. For example, how is mTORC2 regulated and which biological processes does it control? How are the mTORC1 and mTORC2 signalling pathways integrated with each other? What are the functions of these complexes in adult tissues and organs and what are the implications of their dysfunction or dys-regulation in health and disease? Are there additional mTOR complexes that regulate other biological processes? (Ref. Reference Laplante and Sabatini29). And how will RSV affect these pathways?
As RSV enters clinical trials, and becomes more widely available as a supplement, it becomes even more important to understand the mechanisms that lead to both desirable and undesirable effects in vivo. Research into the direct targets and downstream effects of RSV will advance our understanding of cellular biology and help to develop RSV or more specific compounds as potential therapeutic avenues to treat human diseases. Many challenges remain concerning the optimal dosage, since the amounts used in most cell and animal experiments may not be applicable to humans, and multiple distinct targets with differing localisations and sensitivities to the drug are likely to be relevant to health.
Supplementary materials and methods
The Supplementary material referred to in this article can be found online at journals.cambridge.org/erm