Social media summary: Agta hunter–gatherers do not experience age- or sex-related differences in relatedness to their campmates.
Introduction
Across group-living mammals there is a clear association between patterns of social behaviour and the kinship structure of groups, with cooperative behaviours being most highly developed in groups in which the average degree of relatedness is high (Lukas & Clutton-Brock, Reference Lukas and Clutton-Brock2018). Broadly, this is because individuals derive indirect fitness benefits through altruistic behaviours directed towards related individuals; altruism is more likely to be favoured by selection when it occurs among kin (Gardner et al., Reference Gardner, West and Wild2011; Hamilton, Reference Hamilton1964; Lehmann & Keller, Reference Lehmann and Keller2006; West et al., Reference West, Cooper, Ghoul and Griffin2021). In human evolution, all models for the evolution of sociality make some kind of assumption about population structure and relatedness. For instance, differing views about the kinship structure and boundedness of human groups in evolutionary history are embedded in debates about the relative importance of kin vs. group selection in human evolution (Birch, Reference Birch2019). In some cases, assumptions about relatedness are explicit while in other models relatedness and population structure play an important, but unrecognised role (Dyble, Reference Dyble2021). Indeed, many models that have claimed to demonstrate the evolution of cooperation through processes other than kin selection have, in fact, unwittingly rediscovered the role of relatedness (Liao et al., Reference Liao, Rong and Queller2015; Kay et al., Reference Kay, Keller and Lehmann2020).
Given the role of relatedness in the evolution of social behaviour, it is of interest and importance to establish what kind of relatedness and population structure may have been commonplace in human evolutionary history. In order to establish this, it has been typical to reason by ethnographic analogy and to examine the social organisation of contemporary hunter–gatherer societies who probably face many of the same economic and ecological opportunities and constraints as foragers in the past. What patterns of social organisation are typical among contemporary hunter–gatherers? Although there are potential pitfalls to reasoning by ethnographic analogy (Marlowe, Reference Marlowe2005; Page & French, Reference Page and French2020) and delayed-return foraging populations are probably underrepresented in the ethnographic record (Singh & Glowacki, Reference Singh and Glowacki2021), generalising across the available data we can see that contemporary hunter–gatherers typically live in camps in which the majority of co-resident adults are distantly related or unrelated (Hill et al., Reference Hill, Walker, Bozicević, Eder, Headland, Hewlett and Wood2011; Walker & Bailey, Reference Walker and Bailey2014; Walker, Reference Walker2014) and in which households move often between groups and there are not strong sex biases in residence and dispersal (Dyble et al., Reference Dyble, Salali, Chaudhary, Page, Smith, Thompson and Migliano2015; Hill et al., Reference Hill, Walker, Bozicević, Eder, Headland, Hewlett and Wood2011; Wood & Marlowe, Reference Wood and Marlowe2011). Across data compiled in Walker (Reference Walker2014), the mean group relatedness across 34 hunter–gatherer populations (as measured by Wright's coefficient of relatedness, r) was 0.080. These estimates situate humans at relatively low relatedness compared with other highly social mammals living in similarly sized groups (Dyble & Clutton-Brock, Reference Dyble and Clutton-Brock2020) and far from cooperatively breeding species with which humans are sometimes compared (e.g. r = 0.34 among meerkats (Duncan et al., Reference Duncan, Gaynor, Clutton-Brock and Dyble2019), r = 0.46 in Damaraland mole-rats (Burland et al., Reference Burland, Bennett, Jarvis and Faulkes2002) and r = 0.27 among African Wild Dogs (Girman et al., Reference Girman, Mills, Geffen and Wayne1997)). In the more politically stratified hunter–gatherer societies that are argued to be underrepresented in ethnographic data (Singh & Glowacki, Reference Singh and Glowacki2021), the larger group sizes mean that relatedness within groups would probably be lower still, in accordance with the negative relationship between relatedness and group size shown in Walker (Reference Walker2014).
As well as establishing the average degree of relatedness among groups, it is also important to understand age and sex differences in the relatedness of individuals to their group since asymmetries in relatedness to the group may lead to conflicts of interest among group members with consequences for social behaviour (Croft et al., Reference Croft, Weiss, Nielsen, Grimes, Cant, Ellis and Johnstone2021). For example, Cant and Johntone (Reference Cant and Johnstone2008) suggest that the evolution of menopause in humans can be partly explained by an asymmetry in relatedness to offspring between older and younger women that results from female dispersal. Similarly, kin selection modelling by Micheletti et al. (Reference Micheletti, Ruxton and Gardner2017) suggests that sex-differences in relatedness to the group may lead to intrafamilial conflicts of interest with respect to the fitness benefits of engaging in intergroup violence and differences in the benefits of altruistic behaviour towards groupmates Micheletti, Ruxton & Gardner (Reference Micheletti, Ruxton and Gardner2020). Assessing the plausibility of such hypotheses for human social evolution requires more than average relatedness; we also need detailed data on age and sex differences in residence patterns and relatedness to group mates, patterns described by Croft et al. (Reference Croft, Weiss, Nielsen, Grimes, Cant, Ellis and Johnstone2021) as ‘kinship dynamics’.
Another important dimension of relatedness is the distribution of kin across communities and the degree of relatedness between groups. Among hunter–gatherers, having extensive networks of kin across space has been argued to be beneficial to individuals because the ability to move between camps containing kin can provide insurance against local environmental failure (Wiessner, Reference Wiessner2002) and provide opportunities for marrying outside of one's group (Kramer et al., Reference Kramer, Schacht and Bell2017). At a group level, hunter–gatherer social organisation has also been suggested to facilitate cumulative cultural exchange (Hill et al., Reference Hill, Wood, Baggio, Hurtado and Boyd2014; Migliano et al., Reference Migliano, Battiston, Viguier, Page, Dyble, Schlaepfer and Vinicius2020) and, more generally, having tolerant relationships with kin in neighbouring groups has been argued to allow humans to form large multilevel societies (Bird et al., Reference Bird, Bird, Codding and Zeanah2019; Chapais, Reference Chapais2008; Grueter et al., Reference Grueter, Qi, Zinner, Bergman, Li, Xiang and Swedell2020). Although the benefits of cultural exchange and multilevel sociality can be considered emergent or ‘group-level’ benefits, the social organisation that promotes them can be plausibly explained as a by-product of individual-level residential decision making (Dyble et al., Reference Dyble, Salali, Chaudhary, Page, Smith, Thompson and Migliano2015).
Here, we provide a detailed quantitative description of the relatedness within and between residential groups (‘camps’) of the Palanan Agta, a community of foragers from the northern Philippines described below. We explore patterns of relatedness within and between camps, across age and sex, and between spouses. We also assess whether relatedness across groups varies with the degree of population turnover (a proxy for mobility) or engagement in non-foraging economic activities. We find that in camp size and within-camp relatedness the Agta are comparable with previously described immediate-return foraging populations, that there are no age or sex differences in relatedness to the camp, that relatedness between residential camps is low and negatively correlated with distance, and that reduced mobility and engagement in foraging are not correlated with within-group relatedness. These findings provide a detailed quantitative portrait of relatedness among the Agta and show that a bilocal residence system in which either sex may disperse can produce conditions that reduce the potential for conflicts of interest within social groups.
Methods
Ethnographic background
The Agta number around 10,000 people and live in northeastern Luzon, Philippines. The Palanan Agta are a subpopulation of the Agta and live in the municipality of Palanan in Isabela province. As described elsewhere (Minter, Reference Minter2008), the Palanan Agta are presently engaged in a mixed economy dominated by foraging and wet-rice agricultural labour, albeit with substantial differences between camps, with some camps still engaged almost entirely in foraging and others involved largely in agriculture (Dyble et al., Reference Dyble, Thorley, Page, Smith and Migliano2019; Minter, Reference Minter2008). Across the 10 camps included in this study for which time budget data were available, foraging as a proportion of all out-of-camp camp work ranged from 19.5 to 100%, averaging 67%. Foraging among the Agta consists of hunting, spearfishing (both riverine and marine), the gathering of wild plant foods and honey collecting. An estimated 28% of foraged food by weight is traded, usually for rice, increasing the calorific return by ~3 times (Dyble et al., Reference Dyble, Thorley, Page, Smith and Migliano2019; Minter, Reference Minter2008). Food is shared extensively between households, although most smaller items are shared within small clusters of closely related households (Dyble et al., Reference Dyble, Thompson, Smith, Salali, Chaudhary, Page and Migliano2016; D. Smith et al., Reference Smith, Dyble, Major, Page, Chaudhary, Salali and Mace2019). While many Palanan Agta households and camps remain highly mobile, others have become more sedentary (Page et al., Reference Page, Viguier, Dyble, Smith, Chaudhary, Salali and Migliano2016; D. Smith et al., Reference Smith, Dyble, Thompson, Major, Page, Chaudhary and Mace2016). Across the 11 camps included in this study for which data on camp stability can be estimated, this varied from 0.12 to 0.79 and averaged 0.51, where 1 indicates no change in camp membership between our visits and 0 indicates complete turnover.
Previous work on the Palanan Agta has reported average camp size of around 20 adults within which ~25% of adult dyads are primary kin (Dyble et al., Reference Dyble, Salali, Chaudhary, Page, Smith, Thompson and Migliano2015) and in which almost all adults have a kinship connection to at least one other adult in the camp (Dyble, Reference Dyble and Moreau2020). This is consistent with Griffin's (Reference Griffin and Schrire1984) report that the Agta need some kind of kinship tie to an existing camp member in order to join a camp. Mean relatedness among Agta camps has been estimated for the Casiguran Agta as 0.053 (Walker, Reference Walker2014) and for a smaller sample of Palanan Agta communities than in the present study as r = 0.12 (Dyble et al., Reference Dyble, Thorley, Page, Smith and Migliano2019), but note this estimate is inflated in that it is the mean relatedness of camps rather than of individuals to their campmates (see below). Although in principle the Agta prohibit marriage between individuals who can refer to one another by a kinship term (Early & Headland, Reference Early and Headland1998; Minter, Reference Minter2008), exceptions to this are sometimes made (Headland, Reference Headland1987).
Estimating relatedness
Data on relatedness among the Agta were collected during fieldwork in 2013–2014 during which MD, DS and AEP visited all Agta communities in the municipality of Palanan. In every camp visited, we conducted in-depth genealogical interviews with all adults. These interviews were cross-checked and compiled into a genealogy within which our sampled individuals have a mean depth of 3.34 generations (SD = 1.26) and a maximum depth of six generations. As shown by Pemberton (Reference Pemberton2008), genealogies with a depth of three generations will capture the majority of variation in relatedness. We then used the genealogical data to estimate the dyadic relatedness of all individuals using functions from the pedigree and kinship2 packages (Coster, Reference Coster2012; Therneau et al., Reference Therneau, Atkinson, Sinnwell, Schaid and McDonnell2014). We estimated both genealogical relatedness (r), a commonly used measure (Wright, Reference Wright1922), and shared reproductive interest (s) which measures the extent to which an individual will expect to be genetically related to another individual's future offspring, relative to their own offspring. Following Dyble et al. (Reference Dyble, Gardner, Vinicius and Migliano2018) this is defined as s = (r B + r D)/(1 + r C) where r C is the relatedness of the ego to their spouse, r B is the relatedness of ego to alter and r D is the relatedness of ego to alter's spouse. In the absence of consanguineal marriages, s will be identical to r for consanguineal kin but will also capture the shared reproductive interests that exist among affinal kin (Dyble et al., Reference Dyble, Gardner, Vinicius and Migliano2018). For both r and s, we estimated the mean relatedness of individuals to their campmates unless otherwise stated. It is important to note that this is one of three ways in which relatedness could be aggregated. The first option is r group, which is where the relatedness between all group members is averaged separately for each group and then averaged across groups. The second option is r individual, where the average relatedness of each individual to their groupmates is calculated and then averaged across individuals. The third option is r dyads, which is the average relatedness of all co-resident dyads in the sample. The choice of estimate is relevant because where groups differ in size and larger groups are less closely related (as is typical), then r dyads < r individual < r groups. For example, imagine we have two communities: one with 20 individuals all related to each other by r = 0.1 and one with 100 individuals related to each other by 0.02. Mean relatedness within groups (N = 2) is 0.06, mean relatedness of individuals to their group (N = 120) is 0.033 and mean relatedness of co-resident dyads (N = 5080) is 0.023. All three of these measures have some theoretical relevance but r individual is probably the most salient as it determines the indirect fitness benefits that an individual will derive for altruistic behaviours directed towards their group. Previous studies reporting relatedness in human communities are not always explicit about which estimate of relatedness is used although Koster et al. (Reference Koster, Lukas, Nolin, Power, Alvergne, Mace and Massengill2019) and Blurton-Jones (Reference Jones2016) imply use of r individual and Walker (Reference Walker2014) is clear in using r group.
The total sample includes data on 615 individuals from 15 camps including 145 adult men and 126 adult women. These are the same 15 camps analysed in Dyble (Reference Dyble and Moreau2020) and include the 11 camps described in Dyble et al. (Reference Dyble, Salali, Chaudhary, Page, Smith, Thompson and Migliano2015) as well as four camps visited in summer 2014 and not included in the 2015 paper. The categorical composition of these four additional camps is similar to the composition of the 11 camps set out in that paper, with 26.3% (262 of 1004) adult dyads in these four camps being consanguineal (i.e. genetic) kin (vs. 23.9% (924 of 3864) of dyads in Dyble et al., Reference Dyble, Salali, Chaudhary, Page, Smith, Thompson and Migliano2015).
Other variables
Since the Agta do not necessarily know their age in calendar years, estimated age was taken as the mean from a probability distribution of the age of each individual produced by a Gibbs sampling Markov chain Monte Carlo algorithm described in Diekmann et al. (Reference Diekmann, Smith, Gerbault, Dyble, Page, Chaudhary and Thomas2017) that incorporates data from relative age lists produced by informants, broad estimated age brackets from researchers and some known ages that act as ‘anchors’. These estimates were available for all but eight individuals in the study population. These eight individuals were assigned simply as being adults or children. The locations of camps were taken by GPS readings or estimated using Google Earth and distances between camps calculated using functions from the geodist package. Camp stability was measured as in D. Smith et al. (Reference Smith, Dyble, Thompson, Major, Page, Chaudhary and Mace2016) by noting the residents of a camp each time it was visited and calculating the amount of change in camp composition over time, with ‘1’ indicating no change in camp composition and ‘0’ representing complete turnover. Engagement in foraging was measured through the amount of time spent foraging as a proportion of all time spent working out of camp and was assessed using time budget data collected in 10 of the study camps according to the methods set out in Dyble et al. (Reference Dyble, Thorley, Page, Smith and Migliano2019).
Results
Across 271 Agta adults, mean relatedness to all other adults in the population was r = 0.010 (SD = 0.006) and mean relatedness to adult campmates was r = 0.074 (SD = 0.058, Figure 1a). Mean camp size was 18.1 adults (SD = 8.63). Across the 15 camps, camp size was negatively correlated with intracamp relatedness between adults (r = −0.68, p = 0.005, Figure 1c). When relatedness was estimated as the average relatedness of all individuals to all campmates (i.e. including children), mean r was slightly higher at 0.095 (SD = 0.054, N = 615, Figure 1b). Mean relatedness of adults to children in their camp was 0.112 (SD = 0.072, N = 271), and mean relatedness between children was 0.117 (SD = 0.059, N = 344).

Figure 1. Relatedness (r) estimates among the Agta. (a) Histogram of the average relatedness of all 271 adults to their adult campmates; (b) histogram of the average relatedness of all 615 individuals to all their campmates including children; (c) average relatedness between all campmates plotted against camp size for each of the 15 camps; and (d) relatedness of adult women and men to their adult campmates.
As set out above, affinal kin may have shared reproductive interests in the next generation and this can be measured with s, which between adults in the population was, on average, s = 0.016 (SD = 0.011) and between adults and their adult campmates was 0.154 (SD = 0.103). This means that for the average Agta adult, the future offspring of a randomly selected adult campmate is expected – in terms of identity by descent – to be 15% as related to them as they would be to their own future offspring. This, rather than relatedness, is arguably the most salient measure of the fitness benefits that individuals receive from altruism directed towards campmates (Dyble et al., Reference Dyble, Gardner, Vinicius and Migliano2018; cf. Daly & Perry, Reference Daly and Perry2021).
Camp stability, an indicator of the degree of sedentism/mobility, was uncorrelated with camp relatedness, either as a simple correlation (r = −0.077, p = 0.82, n = 11) or as a partial correlation controlling for camp size (r = −0.20, p = 0.57). The proportion of out of camp work time that camp members engaged in foraging was correlated with camp relatedness (r = 0.64, p = 0.047), but this was not preserved in a partial correlation controlling for group size (r = 0.14, p = 0.71); camps engaged in more market-integrated activities tended to be larger but no more or less closely related than expected given their size.
Sex and age differences in relatedness to the camp
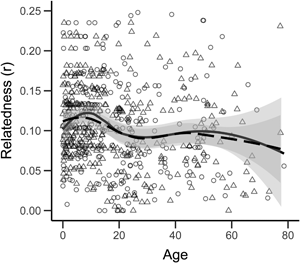
We see no sex difference in the average relatedness of adults to their adult campmates: men, r = 0.076 (SD = 0.059, N = 145); women, r = 0.073 (SD = 0.058, N = 126); two-tailed permutation test, p = 0.694; Figure 1d). Similarly, we see no sex difference in the average degree of shared reproductive interest between adults and their adult campmates: men, s = 0.152 (SD = 0.097, N = 145); women, s = 0.155 (SD = 0.111, N = 126); two-tailed permutation test, p = 0.798). In contrast to many non-forager societies where relatedness varies with sex and age (Koster et al., Reference Koster, Lukas, Nolin, Power, Alvergne, Mace and Massengill2019), our cross-sectional analysis suggests that relatedness to campmates remains relatively constant throughout adult life for both men and women (Figure 2a), albeit with a slight reduction in the relatedness of both men and women to their adult campmates from 20 to 30 years of age associated with dispersing to marry and a subsequent increase as adult children begin to be included as campmates (Figure 2b). Similar results are obtained for estimates of shared reproductive interest (Figure 2c and d).

Figure 2. Relatedness and shared reproductive interest of individuals to campmates by age and sex for (a) r of all individuals to all campmates (including children), (b) r of adults to adult campmates, (c) s of all individuals to all campmates (including children) and (d) s of adults to adult campmates. Boys/men are triangles, girls/women are circles. Lines are LOESS curves with 0.5 sensitivity and grey bands show the standard error. Solid lines and solid bands are for girls/women and dashed lines and dotted bands are for boys/men.
Distribution of kin across camps
There is a negative correlation between the relatedness between adults from each pair of camps and the distances between those camps (Spearman r = −0.51, p < 0.001, Figure 3a). The combination of a near absence of close consanguineal marriage and the bilocal residence means that Agta kinship networks are relatively diffuse and, on average, adults had consanguineal kin of at least r = 0.0625 (equivalent to a cousin's child) in 4.07 of the 15 study camps (SD = 2.58, range 0–11, N = 271 adults). Note that many of these adults will also have kin in camps outside of the municipality where data collection was focused, such that the full extent of their kin network is much greater. Although women had, on average, kin living in a slightly larger number of camps (men, mean = 3.87, SD = 2.43, N = 145; women, mean = 4.29, SD = 2.74, N = 126, Figure 3b), this difference is not significant (permutations test, p = 0.088, Figure 3b). Affinal relationships also play a role in extending kinship networks across camps: by virtue of being able to reside with male and female kin of both the husband and wife, the average household can reside in 6.70 of the 15 camps (SD = 2.94, range 1–14, N = 158).

Figure 3. (a) Scatter plot showing the average adult relatedness and distance between each pair of camps (N = 105 pairs); (b) histogram of the distribution of number of camps containing consanguineal kin (r ≥ 0.0625) for women and men; and (c) jitter plot showing the estimated age differences between Agta spouses.
Consanguinity and age differences between spouses
As noted above, the Agta have rules against marrying kin. Although Headland (Reference Headland1987) suggests that in practice these rules are pragmatically interpreted and Minter (Reference Minter2008) suggests that rates of consanguineous marriage (i.e. marriage between close genetic relatives) may be increasing, our data show that such marriages remain rare among the Palanan Agta. Of 80 marriages, 78 had no known shared genealogical ancestry, one couple were second cousins (r = 0.03125), and one couple were first cousins (r = 0.125; the husband's mother and wife's father are full siblings). As an additional analysis, we looked at the age differences between Agta spouses. Of the 78 marriages where we had an age estimate for both individuals, the husband was older in 67 marriages (86%) and the wife was older in 11 (14%) (Figure 3c). The average difference between husband age and wife age (calculated as husband age – wife age) was 4.29 years (SD = 5.31) and ranged from −7.1 to 32.7 years. This maximum difference of 32.7 years was between a 17-year-old woman and 50-year-old man and was a large outlier; the second largest difference was 13.1 years.
Discussion
Here, we have provided a quantitative description of the relatedness structure within and between Agta residential camps. These data show sex equality in Agta residence, with no sex difference in either relatedness to the camp or the distribution of kin across residential camps. The results also show that, on average, the relatedness of adults to their camp mates does not change with age. This is in contrast to what is seen in populations in which one sex disperses and increases their relatedness to their group with age (Koster et al., Reference Koster, Lukas, Nolin, Power, Alvergne, Mace and Massengill2019). From an inclusive fitness point of view, there are no asymmetries in the benefits that any sex or age class of individual will derive from cooperating with their campmates. This is significant because asymmetries in relatedness between men and women of different ages are a critical assumption in many models of social evolution (Croft et al., Reference Croft, Weiss, Nielsen, Grimes, Cant, Ellis and Johnstone2021). For example, Cant and Johnstone's (Reference Cant and Johnstone2008) model of reproductive conflict assumes that female-biased dispersal results in older women being more closely related to the children of younger women (their son's wives children) than younger women are to the children of older women (their husband's mother's children). Under these conditions and in the event of reproductive competition, younger women would be insensitive to costs felt by older women, potentially leading to early cessation of reproduction. The female-biased residence required for the evolution of menopause in this model is not seen in our data and is not typical of immediate-return hunter–gatherers more broadly (Hill et al., Reference Hill, Walker, Bozicević, Eder, Headland, Hewlett and Wood2011).
The mean relatedness between adults across the whole population of the Palanan Agta of r = 0.01 is similar to that reported for the Hadza (Blurton Jones, Reference Jones2016) and the average relatedness of adults to their adult campmates of 0.074 is also similar to previously described estimates for hunter–gatherers: Walker (Reference Walker2014) estimates a mean r of 0.080 across 34 hunter–gatherer groups and Burton-Jones estimates r = 0.0753 among the Hadza (Blurton Jones, Reference Jones2016). What determines the relatedness of these groups? As we have argued previously (Dyble et al., Reference Dyble, Salali, Chaudhary, Page, Smith, Thompson and Migliano2015), group relatedness is likely to be constrained under bilocal residence, as seen among the Agta and bilocal hunter–gatherers who tend to live in groups of lower relatedness than horticultural or agricultural groups with sex-biased dispersal, even if controlling for group size (Dyble et al., Reference Dyble, Salali, Chaudhary, Page, Smith, Thompson and Migliano2015; Walker, Reference Walker2014). More generally, important features of human demography and life history such as monotocy (the production of single offspring per pregnancy) and low reproductive skew are also predicted to reduce group relatedness (Dyble & Clutton-Brock, Reference Dyble and Clutton-Brock2020). Whether our estimates of relatedness within groups are based on hunter–gatherers or other societies, these estimates situate humans at relatively low relatedness compared with other highly social mammals living in similarly sized groups (Dyble & Clutton-Brock, Reference Dyble and Clutton-Brock2020) and similar to estimates from chimpanzees (Langergraber et al., Reference Langergraber, Mitani and Vigilant2007). As such, even if humans can be reasonably argued to be cooperative breeders (e.g. Hrdy, Reference Hrdy2007; Kramer, Reference Kramer2010; Van Schaik & Burkart, Reference van Schaik and Burkart2010) then the evolutionary pathway that led to this is probably very different from the pathway to cooperative breeding in other mammals based on a high degree of within-group relatedness (Lukas & Clutton-Brock, Reference Lukas and Clutton-Brock2012, 2018).
The low frequency of consanguineal marriage seen among the Palanan Agta is also typical of contemporary hunter–gatherer societies, and in contrast to the greater frequency of consanguineal marriage seen among agropastoralists (Bailey et al., Reference Bailey, Hill and Walker2014; Walker & Bailey, Reference Walker and Bailey2014). In combination with bilocal residence, these low rates of kin marriage mean that the Agta have diffuse networks of kin across camps of the kind that have been argued to have been important in buffering individuals against local environmental failure (Wiessner, Reference Wiessner1977), in facilitating the exchange of goods and cultural ideas (Dyble, Reference Dyble2018; Hill et al., Reference Hill, Wood, Baggio, Hurtado and Boyd2014; Migliano et al., Reference Migliano, Battiston, Viguier, Page, Dyble, Schlaepfer and Vinicius2020) and in increasing the availability of marriage partners in groups otherwise susceptible to random fluctuations in adult sex ratio (Kramer et al., Reference Kramer, Schacht and Bell2017).
Overall, our results show that in terms of camp size, residence patterns and within-group relatedness, the Palanan Agta are similar to other immediate-return hunter–gatherer communities including the Ache, Hadza and Ju/’hoansi (Blurton Jones, Reference Jones2016; Hill & Hurtado, Reference Hill and Hurtado1996; Hill et al., Reference Hill, Walker, Bozicević, Eder, Headland, Hewlett and Wood2011; Walker, Reference Walker2014). On one hand, the similarities in social organisation between hunter–gatherer societies living on different continents and in diverse environments represent remarkable consistency, lending weight to the use of the ethnographic analogy to reconstruct the social organisation of foragers in the past and to the foundational assumption of human behavioural ecology that convergence in lifeways will occur in response to similar subsistence challenges. However, it is clear that contemporary immediate-return hunter–gatherer societies are not representative of all hunter–gatherers in the past because the existing sample may preferentially be seen in marginal environments unsuitable for farming (Cunningham et al., Reference Cunningham, Worthington, Venkataraman and Wrangham2019; Marlowe, Reference Marlowe2005; Porter & Marlowe, Reference Porter and Marlowe2007), because a focus on immediate-return foragers neglects the more marked social and political complexity and inequality seen in many delayed-return and sedentary foragers, usually associated with defensible resources (Moreau, Reference Moreau and Moreau2020; Ringen et al., Reference Ringen, Martin and Jaeggi2021; E. A. Smith & Codding, Reference Smith and Codding2021), and because contemporary hunter–gatherers have important relationships with neighbouring non-foraging groups and wider state societies that may have influenced their way of life (Headland et al., Reference Headland, Reid, Bicchieri and Munoz1989; Lee & Guenther, Reference Lee and Guenther1991; Singh & Glowacki, Reference Singh and Glowacki2021; Wilmsen, Reference Wilmsen1989). Therefore, even if the kind of social organisation seen among contemporary hunter–gatherers is a good model for some foraging societies in human evolutionary history, it is unlikely to have been some kind of ‘universal’ form. However, as our results demonstrate, when economic and environmental conditions do facilitate mobility and bilocal residence, individuals may vary very little in their relatedness to camp mates by either sex or age. Although relatedness is not the only determinant of the fitness benefits of cooperation, it plays an important role and a lack of asymmetry in relatedness may promote the egalitarian political systems seen among the Agta and many other immediate-return foraging societies (Boehm, Reference Boehm2009; Kelly, Reference Kelly2013; Woodburn, Reference Woodburn1982).
Acknowledgements
We thank the Palanan Agta and the Curampez family for their kindness and hospitality.
Author contributions
MD, AEP and DS collected data. ABM conceived of the project in general and MD of this study in particular. MD performed statistical analyses and wrote the paper with contributions from all authors.
Financial support
A.B.M. received funding from the Leverhulme Trust (grant no. RP2011-R 045).
Conflicts of interest
The authors have no conflicts of interest to declare.
Data availability
The data and code are available as Supplementary Materials.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ehs.2021.46