Deep brain stimulation (DBS) of the globus pallidus (GPi) or subthalamic nucleus (STN) is an established treatment for dystonia in primary conditionsReference Vidailhet, Vercueil and Houeto1, Reference Volkmann, Wolters and Kupsch2 or when it is associated with other neurodegenerative disorders, such as Parkinson’s disease (PD).Reference Gurevich, Peretz and Moore3 DBS is particularly indicated when medications or botulinum toxin (BoNT) have failed to provide adequate treatment. Although DBS is highly effective, patients often continue to display dystonic features even after surgery for a series of reasons. In fact, dystonia might not fully respond to DBS in specific body sites or it only resolves with stimulation parameters causing side effects; furthermore, dystonia can be caused by DBS itself.Reference Baizabal-Carvallo and Jankovic4
BoNT serotype A (BoNT-A) has been shown to be beneficial in the treatment of focal and segmental dystonia, with confirmed clinical effectiveness and reasonably safe tolerability profile.Reference Albanese, Barnes and Bhatia5, Reference Ricciardi, Bove and Fasano6 Expert opinion-based guidelines recommend the use of BoNT-A in DBS patients in order to treat dystonia in an effective, safe, and focal fashion.Reference Albanese, Barnes and Bhatia5, Reference Hallett, Albanese and Dressler7 To this date, no study has investigated the effectiveness of the combined use of DBS and BoNT.
IncobotulinumtoxinA (Xeomin®, Merz Pharma, Frankfurt am Main, Germany) is a BoNT-A complex that differs from other formulations in that it does not have accessory proteins. The significance of this has yet to be fully established although clinical experience confirms its less immunogenicity.Reference Ricciardi, Bove and Fasano6 IncobotulinumtoxinA studies of patients with blepharospasm and cervical dystonia have shown it to be non-inferior to OnabotulinumtoxinA (Botox®, Allergan, Irvine, CA, USA) with a 1:1 dose ratio.Reference Hallett, Albanese and Dressler7
In this retrospective study, we sought to report the usefulness of IncobotulinumtoxinA in DBS patients. We hypothesized that the combination of DBS and BoNT-A is safe and synergic when used in patients with dystonia. In addition, in patients also receiving OnabotulinumtoxinA, we compared the treatment outcome of the two BoNT-As.
This is a retrospective single-center chart-review of patients seen at the Movement Disorder Centre of the Toronto Western Hospital from 1995 to 2017. Keywords “botulinum toxin,” “botox,” “xeomin,” and “deep brain stimulation” were used in our records search. Inclusion criteria was having undergone DBS and at least one treatment with IncobotulinumtoxinA at any given point in time. The primary outcome was to compare the use, effectiveness, and safety of IncobotulinumtoxinA before and after DBS. Secondary outcome was the comparison between IncobotulinumtoxinA and OnabotulinumtoxinA in patients receiving both.
The following data were retrieved and entered into a standard form: demographic data (age, gender, underlying patient condition, disease duration) and number of treatments. For each treatment, the following data were also recorded: date and number of treatment sessions, type of BoNT-A injected (IncobotulinumtoxinA or OnabotulinumtoxinA), total dose and dilution, and number of sites injected. The severity of dystonia was assessed by dedicated rating scale scores and efficacy of treatment was also expressed by qualitative assessment of duration of effect and Clinical Global Impression (CGI), assessing improvement and efficacy (CGI-I and CGI-E, respectively).Reference Busner and Targum8 Lastly, any side effects were also documented.
Due to the small sample size, non-parametric Wilcoxon and Mann–Whitney tests were used for paired and unpaired comparison. The test was considered significant when the p-value was <0.05.
Our search identified 68 patients of which 36 patients met the inclusion criteria. Sufficient clinical information was available for 25 of them (13 females; mean age: 59.4 ± 14.1 years). Their underlying conditions include PD (n: 17), cervical dystonia (n: 5), generalized dystonia (n: 2), and multiple sclerosis tremor (n: 1). The average disease duration and disease rating scales/scores (at the time of undergoing DBS) are shown in Table 1. Indications for BoNT-A treatment were cervical dystonia (n: 10), blepharospasm (n: 7), lower limb dystonia (n: 7), sialorrhea (n: 5), and trunk dystonia (n: 2).
Table 1: Demographics of patients included in the study
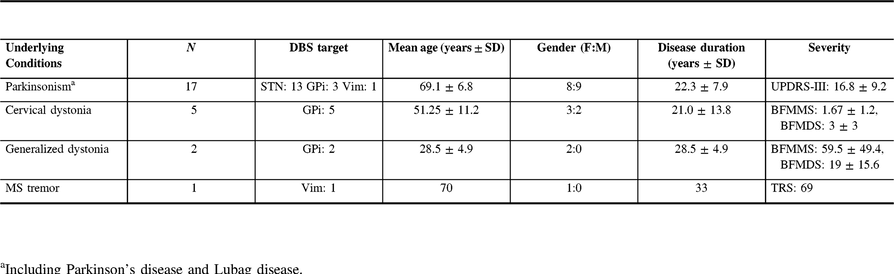
BFMDS=Burke–Fahn–Marsden Disability Scale; BFMMS=Burke–Fahn–Marsden Movement Scale; GPi=globus pallidus pars interna; MS=multiple sclerosis; STN=subthalamic nucleus; TRS=Fahn Tolosa Marin Tremor Rating Scale; UPDRS=Unified Parkinson Disease Rating Scale; Vim=ventral intermediate thalamic nucleus.
a Including Parkinson’s disease and Lubag disease.
Five patients underwent a total of 20 sessions of IncobotulinumtoxinA before DBS. Indications were cervical dystonia (n: 2), generalized dystonia (n: 2), and foot dystonia (n: 1). They had all ceased injections at the time of evaluation: two patients ceased injections before surgery due to lack of benefit, whereas three patients continued to have IncobotulinumtoxinA treatment until optimization of DBS settings. The mean number of units used for IncobotulinumtoxinA was 252.5 ± 120.6, mean number of sites per session was 9.8 ± 5.4, mean duration of effect was 5.1 ± 2.9 weeks, mean CGI-I was 2.1 ± 1.1, and mean CGI-E was 5.6 ± 4.3.
Twenty patients had a total of 282 treatment sessions with BoNT-A after undergoing DBS: 125 sessions using IncobotulinumtoxinA and 157 using OnabotulinumtoxinA. Indications were cervical dystonia (n: 7), blepharospasm (n: 6), lower limb dystonia (n: 6), and sialorrhea (n: 5). The number of sessions, units used, sites per session, duration of effect, and overall effect according to CGI-I and CGI-E for each indication are shown in Table 2. There was an overall moderate effect (CGI-I) of IncobotulinumtoxinA (2.0 ± 0.9) with minimal side effects (CGI-E: 5.1 ± 3.8). The average duration effect was 7.1 ± 4.1 weeks. The blepharospasm group required the most number of injection sites per session (10.2 ± 2.5) and the lower limb dystonia group required the highest number of total units used per session (192.4 ± 131.4).
Table 2: Number of sessions, units, sites, duration of effect, and CGI-I and CGI-E according to indication for all patients using IncobotulinumtoxinA after DBS

DBS=Deep brain stimulation; CGI-I=Clinical Global Impression Scale – Improvement; CGI-E=Clinical Global Impression Scale – Efficacy.
a Some patients received BoNT-A for more than one indication.
At the time of data collection, eight patients had ongoing treatment and 12 patients had ceased the treatment due to lack of benefit (n: 5), DBS-related improvement of symptoms (n: 2), and side effect (headache in one patient). There was no clear documentation for IncobotulinumtoxinA discontinuation in remaining four patients.
There was no significant difference when comparing the use of IncobotulinumtoxinA before and after DBS when looking at the number of sessions, mean number of sites of injection used, average duration of effect, and CGI-I and CGI-E. However, there was a highly significant difference between groups with the number of units used when all conditions were analyzed all together (252.5 ± 120.6 pre-DBS vs. 118.9 ± 99.1 post-DBS, p < 0.001).
With respect to indications, statistical comparison could only be made when IncobotulinumtoxinA was used for cervical dystonia due to small sample size. There was a statistical reduction in the number of units used (290.7 ± 124.0 vs. 192.4 ± 131.4, p = 0.005) and an increased duration effect (4.0 ± 3.4 vs. 8.4 ± 3.6 weeks, p = 0.003).
Fourteen of the 20 patients who received IncobotulinumtoxinA also had OnabotulinumtoxinA treatment. Nine patients were switched to IncobotulinumtoxinA due to no benefit from OnabotulinumtoxinA (7), worsening gait (1), and lack of reimbursement (1). Five patients were switched to OnabotulinumtoxinA due to unknown reasons.
In the eight patients with blepharospasm and in the six patients with cervical dystonia, there was no significant difference in all treatment features except in the number of sites used in cervical dystonia patients (10.6 ± 2.5 with IncobotulinumtoxinA vs. 9.9 ± 2.6 with OnabotulinumtoxinA, p-value 0.0494) (Table 3).
Table 3: Group of patients that have used both IncobotulinumtoxinA and OnabotulinumtoxinA post-DBS
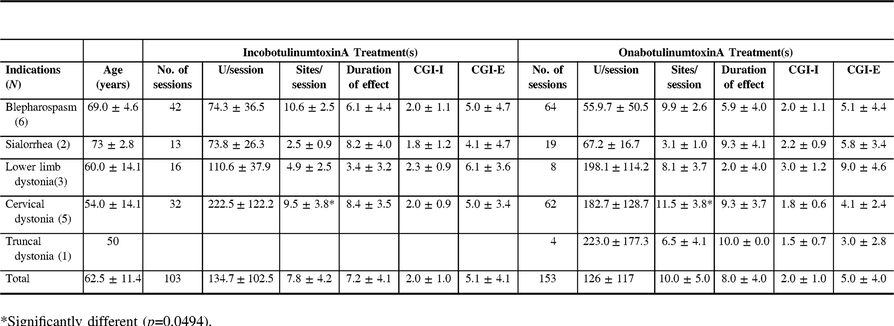
DBS=Deep brain stimulation; CGI-I=Clinical Global Impression Scale – Improvement; CGI-E=Clinical Global Impression Scale – Efficacy.
* Significantly different (p=0.0494).
This retrospective study has shown that the combined use of IncobotulinumtoxinA and DBS is a useful strategy for patients not achieving adequate symptomatic control with DBS alone. Our results show an equivalent efficacy and safety use of IncobotulinumtoxinA before and after surgery with a significant difference in the total number of units used, thus suggesting that there is a synergistic effect of DBS and IncobotulinumtoxinA. Findings also show that IncobotulinumtoxinA has effect and safety similar to other studies: a latency of 1–2 weeks and a peak effect at 8 weeks and moderate improvement in GCI scale.Reference Hallett, Albanese and Dressler7, Reference Rieu, Degos and Castelnovo9
There is very little evidence of the combined safety and efficacy of DBS and BoNT-A and no evidence currently comparing the effectiveness of BoNT-A before and after DBS. A short report in a group of six patients with foot dystonia post-DBS has shown that there was an improvement with OnabotulinumtoxinA, with a duration effect of approximately 5 months,Reference Gupta and Visvanathan10 a figure substantially longer than in our cohort, the longest being 8.4 weeks. Increasing dose of BoNT-A may have resulted in longer duration of effect.
Previous studies have shown that there is no difference of efficacy in IncobotulinumtoxinA and OnabotulinumtoxinA in the treatment of blepharospasm, cervical dystonia, and leg dystonia.Reference Hallett, Albanese and Dressler7 This was also seen in our results.
There are several limitations to this study, such as the small sample size (before and after DBS) and the non-blinded and retrospective analysis. In addition, patients receiving BoNT-A before and after DBS were not the same, further challenging our hypothesis that these two treatments are synergic. Furthermore, the lack of dedicated rating scales before and after treatment and a formal quantitative assessment of duration effect after each treatment represent other limitations. Lastly, results with OnabotulinumtoxinA have to be interpreted with caution as it was not the main focus of the study and we collected incomplete data for patients using OnabotulinumtoxinA.
In conclusion, in spite of the limits of our retrospective study, we suggest that IncobotulinumtoxinA is useful to use in conjunction with DBS and the availability of BoNT-A as treatment after DBS should not be forgotten. There exists a possibility that DBS may have an additive effect on patients needing BoNT-A and may be useful in patients that were previously resistant to BoNT-A. A prospective double-blind study is certainly needed for patients who have undergone DBS, a population excluded from BoNT-A trials thus far.
Disclosures
The study was funded by a grant from Merz. DS has nothing to disclose. AF reports grants, personal fees, and non-financial support from Abbvie; grants, personal fees, and non-financial support from Boston Scientific; grants, personal fees, and non-financial support from Medtronic; personal fees from Chiesi; personal fees and non-financial support from Ipsen; personal fees from UCB; and grants and personal fees from Sunovion, outside the submitted work.
Statement of Authorship
DS: execution and writing of the first draft. AF: conceptualization of the study, revising the manuscript, and final approval of the version to be published.







