Introduction
Saunders's Gull Larus saundersi is recognized as ‘Vulnerable’ by IUCN (2008) and Birdlife International (2008), and is also listed as vulnerable in China, but it is not nationally protected due to a lack of available information (Zheng and Wang Reference Zheng and Wang1998). Its status and distribution have been a mystery for more than a century since its initial description (Swinhoe Reference Swinhoe1871). Following the proposal by Melville (Reference Melville1987), studies on Saunders's Gull were given more attention by ornithologists around the world. The current world population estimate is a minimum of 14,400 birds, which is more than 70% higher than the mid-point of the estimated range (7,100–9,600) by Wetlands International (2006). However the apparent population increase is almost certainly due to increased survey effort. It is likely that the population is continuing to decline, given the significant threats to habitats and high human disturbance levels occurring across the species's range (Cao et al. Reference Cao, Barter and Wang2008).
Saunders's Gull is restricted to Common Seepweed Suaeda glauca habitats for breeding on the east coast of China and South Korea, many of which have been lost and degraded due to human land use (Shi et al. Reference Shi, Thouless and Melville1988, Huang Reference Huang1994, Moores Reference Moores2002). Yancheng National Nature Reserve (Yancheng NNR) is one of three major breeding and wintering areas in China (Hou et al. Reference Hou, Chu, Qian, Lu and Dai2000). It consists of coastal wetlands across five counties in Jiangsu Province. In recent years, some tidal marshes have been converted to aquaculture ponds, salt pans, agriculture, industrial development and other land use types. Meanwhile, since the introduction of the alien Smooth Cordgrass Spartina alterniflora into Yancheng coastal wetland in 1982 (Zhong et al. Reference Zhong, Zhou and Zhou1985), the plant has expanded into the open mudflats and occupied the periphery of the Common Seepweed community from Sheyang River to Liangduo River (Liu et al. Reference Liu, Zhang, Jiang and Wang2009a). This has seriously affected conditions for breeding, wintering and migratory waterbirds including Red-crowned Cranes Grus japonensis, Black-faced Spoonbill Platalea minor, ducks and waders (Nanjing Environment Sciences Institute et al. 2005).
In this paper, we measure changes in habitats of Yancheng coastal wetlands, especially habitats suitable for breeding Saunders's Gulls, through interpretation of satellite images from six different years, from the establishment of the National Nature Reserve in 1992 to 2007. Combined with ground survey data on the size and distribution of the population and nests, we analysed the actual and potential impact of changes in breeding habitat on the breeding population size and distribution, as well as that of nest-sites. Finally, we also put forward some measures for species conservation and habitat management.
Study area
Yancheng NNR (Figure 1) is located in the centre of the east coast of China, between 32°38′03″ and 34°30′08″N, 119°51′25″ and 121°05′47″E (the former reserve ranged between 32°34´ and 34°31´N, 119°48´ and 121°15´E). The major objective of the reserve is to protect the Red-crowned Crane and other bird species as well as their habitats. It was approved as a National Nature Reserve by the China State Council and internationally recognised as a Biosphere Reserve by UNESCO in 1992 (Han and Gretchen Reference Han and Gretchen1995). Due to the accretion of mudflats and anthropogenic impacts, the functional zones were adjusted and approved by China's State Council in early 2007. The current reserve area is 284,179 ha, a decrease of 169,321 ha in comparison with the former reserve (Nanjing Environment Sciences Institute et al. 2005).
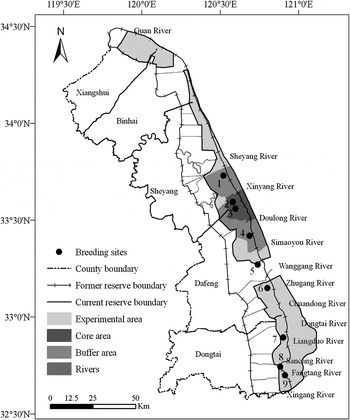
Figure 1. Nesting area distribution and revised functional areas of Yancheng NNR, Jiangsu Province. 1: Dongshagang, 2: North of core Area, 3: South of core area, 4: North of Simaoyou, 5: North of Wanggang, 6: North of Zhugang, 7: North of Liangduo River, 8: South of Sancang River, 9: South of Fangtang River.
The boundaries of the study area followed the former reserve boundaries except that the eastern boundary is in accordance with that of the current reserve. The area of the study area is 579,136 ha (Figure 1).
Methods
Field methods
Saunders's Gulls spend the summer from March to September in Yancheng NNR. In our study, the number and distribution of Saunders's Gulls in Yancheng NNR was surveyed in early June of each year when they are in the stable part of the breeding cycle. The gulls were counted by one person standing on a high point while the other survey members went into nesting areas. As this is a colonial nesting species (Du Reference Du1994), we searched thoroughly for nests in each Common Seepweed habitat between April and July 1999–2007. Due to the occurrence of avian influenza, field work was not conducted in 2004. All nests were recorded using a GPS and marked 1 m to the south with a surveyor's flag rolled up and pushed into the ground leaving 4–5 cm of flag exposed to monitor productivity and also to avoid duplicate recording of nest sites.
Processing of satellite imagery
Cloud-free Landsat TM images for 1992, 1995, 1999, 2002, 2005 and 2007 were used for habitat classification (image orbits for each year include 120/36 and 119/37), which were acquired from February to March since this is the dry season in the study area. Ancillary GIS data used in this research included a digital topographic map of Yancheng NNR from 1986 at the scale of 1:50,000.
All images were registered in a Gauss projection (identical to that of the topographic map) using ERDAS software. Between 50 and 60 ground control points (GCPs) were used for each image, which were evenly distributed throughout the whole study area. The registration procedure achieved an accuracy of less than 0.5 pixel rms error (RMSE) for images in 1992, 1995, 1999, 2002, 2005 and 2007.
Our study area runs across two TM images, so a mosaic was made in the pre-processing stage. We performed histogram matching between the adjacent images for the same year using ERDAS Imagine 8.7 software to conduct image-to-image radiometric normalisation, as suggested by Tang et al. (Reference Tang, Wang and Zhang2005).
To establish visual interpretation marks, ground-truth surveys were conducted over the study area in order to collect real time ground-truth data between February and March in 2005 and 2007 with the help of a global positioning system (GPS). Reference data were collected extensively within the study area and vegetation cover was identified in the field. The accuracy of the resulting landscape maps was assessed using field survey reference data from the study area in 2005 and 2007. Their error matrix indicates that the overall accuracy exceeded 90%.
Classification of habitat types
The sequence of the natural vegetation succession from the sea in a landward direction is: mudflats, Smooth Cordgrass, Common Seepweed, grassland and Common Reed Phragmites communis (Ma et al. Reference Ma, Wang and Tang1999). We classified the coastal wetlands into 14 habitat types: residential area (RA), industrial land (IL), farmland (FL), forests (FR), reed (RD), grassland (GL), aquaculture ponds (AP), reservoirs (RE), salt pans (SP), rivers (RV), Common Seepweed (CS), Smooth Cordgrass (SC), mudflats (MF) and lower mudflats (LM). Meanwhile, we defined RD, GL, CS, SC, MF and LM as natural wetlands, and AP, RE, SP and FL as anthropogenic wetlands.
Data processing
All vector data were converted to raster data on a 100 m × 100 m grid scale. All spatial data were managed using ArcGIS 9.1. We used the Spatial Analyst tool to generate the habitat transition matrix between different years. The centroid coordinates of Common Seepweed and Smooth Cordgrass habitats were acquired using Mean Centre Function of Measuring Geographic Distributions in Spatial Statistics Tools, and the transition angle and distance were acquired by the Near Function analysis tools.
Using the point density function in Spatial Analyst, the annual nest density figure was generated and the number of nests in each cell was also obtained. We defined a minimum rectangle of 37,807 ha (the coordinates of the centroid are N33.56329° and E120.58250°) to cover all nest-sites in the south of core area (site code 3 in Figure 1) during 1999-2007. The overall nest density figure was generated by adding the values of all rasters on a cell by cell basis. Proportions of abandoned nest-sites and available sites occupied between consecutive years (rates of nest-site turnover) were calculated for each nest area from nest-site turnover rate = [0.5(S1 ÷ N1)+(S2 ÷ N2)], where S1 = number of sites occupied only the first year, N1 = total number of sites occupied the first year, S2 = number of sites occupied only the second year, and N2 = total number of sites occupied the second year (Eileen Reference Eileen1996).
All statistics were preformed using SPSS 11.5 software. All means are presented ± Standard Error (SE) with the range from minimum to maximum presented in brackets.
Results
Population size and distribution of Saunders's Gull
Annual mean population size and breeding population size were 994.09 ± 170.17 individuals (n = 11,575–1,300 individuals) and 874.09 ± 189.49 (n = 11, 575–1,300) separately across the eleven-year period (Table 1). Except in 2007, the total population size was > 900 individuals. The mean breeding population size in the core area of the reserve was 685.18 ± 55.69 individuals (n = 11,377–902), which accounted for 81.82% ± 7.62% (36.82%–100%) of the total breeding population in 1992–2007. The number of breeding sites decreased from eight in 1992 and 1994 (Huang Reference Huang1994, Wang and San Reference Wang and San1994) to four in 1998 and a single site in 2000–2006 (Figure 2). Furthermore, in 2007, one new breeding site was observed. However, the total population size was only 575 gulls, which represented a decrease of about 45% in comparison with the earlier years.

Figure 2. Changes in breeding population size and number of breeding sites of Saunders's Gull at Yancheng NNR 1992-2007.
Table 1. Numbers and distribution of Saunders's Gull in coastal marshes of Yancheng NNR, 1992–2007.

Notes:
Data from 1992, 1994 and 1998 were sourced from Huang (Reference Huang1994), Wang and San (Reference Wang and San1994) and Chu et al. (Reference Chu, Hou, Qian, Liu and Wang2000) respectively. The remaining data were collected during the field survey by the authors. The breeding population size is shown in parentheses. Site codes are as in Figure 1.
Changes in land use and land cover
The land cover classification clearly indicated that large areas of Common Seepweed have been lost along the beach from Sheyang River to Liangduo River except in the core of Yancheng NNR in the study area and that fragmentation of Common Seepweed habitats has occurred elsewhere. Calculations by each LULC class for 1992 and 2007 (Figure 3) showed that 79.1% (27,358 ha) of Common Seepweed has been lost within this 15-year period, while the area occupied mainly by aquaculture ponds more than doubled (122.4% increase) during the same period. However, the introduced Smooth Cordgrass habitat increased in area by 321.9% (11,057 ha) from Sheyang River to Liangduo River from 1992 to 2007 (Figure 3). This mainly occupied the mudflats and constrained the natural transition of tidal vegetation, particularly the Common Seepweed community. The results also showed that land reclamation mostly occurred in Common Seepweed habitats in 1992–2002 and shifted to reclaim the Smooth Cordgrass habitats afterwards (Figure 3). The area of reed and mudflats decreased by 76.1% (30,686 ha) and 16.5% (15,810 ha) respectively, and that of farmlands increased by 12.0% (28,213 ha) within this 15-year period.

Figure 3. Changes between 1992 and 2007 in the area of five main types of land use or land cover in Yancheng coastal wetlands, Jiangsu (AP: Aquaculture ponds, FL: Farmland, SC: Smooth Cordgrass, CS: Common Seepweed, RD: Reed).
Land reclamation is the major cause of the loss of breeding habitats of Saunders's Gull. Seven breeding sites observed in 1992 and 1994 were lost due to land reclamation and were converted to aquaculture ponds, farmland and industrial use (Table 2).
Table 2. Date and causes of the loss of breeding sites of Saunders's Gull at Yancheng NNR (Site codes as in Fig. 1).

Changes in Common Seepweed and Smooth Cordgrass communities
The number of patches (NP) and total area of the Common Seepweed community decreased continuously from 1992 to 2007 (Figure 4). However, the mean patch area did not change significantly (P > 0.05). This represented a decrease of 88.31%, and conversion to aquaculture ponds was the main cause of Common Seepweed loss (Table 3). There was a significant difference in the area of this plant converted between 1992 and 2007 (t 4 = 3.491, P < 0.005), and its centroids shifted towards the south-east of the study area (Figure 5).

Figure 4. Area and number of patches of Common Seepweed and Smooth Cordgrass habitats in the study area 1992–2007.

Figure 5. Mean centres of Common Seepweed and Smooth Cordgrass communities in Yancheng NNR 1992–2007.
Table 3. (a,b) The change in the area of (a) Common Seepweed and (b) Smooth Cordgrass habitats in Yancheng costal wetlands over five periods of varying length between 1992 and 2007. The extent of habitat loss or gain is expressed as the percentage change of the area at the beginning of each period.

The NP of Smooth Cordgrass increased between 1992 and 1995, declined to 2002 and then increased in 2002–2007 (Figure 4). However, the area of Smooth Cordgrass increased significantly (P < 0.05). This represented an increase of 391.56%, and 92.83% of the increased area was converted from the mudflats in this period (Table 3). The centroids of the Smooth Cordgrass community shifted towards the east (Figure 5).
Number of nests and density of Saunder's Gull
The number of nests in Zhonglugang tended to decrease and that of Sanlizha to increase (Table 4), with significant differences across years at Zhonglugang: (t 7 = 6.29, P < 0.01) and Sanlizha (t 7 = 3.04, P < 0.02), and differences within a year but between two sites (t 7 = 3.537, P < 0.01). The overall nest density map (Figure 6) indicated that 398 cells contained 1–2 nests, 200 cells contained 3–8 nests and 20 cells contained 9–16 nests.

Figure 6. Nest-site density of Saunders's Gull in the south of core area of Yancheng NNR 1999–2007.
Table 4. The number and density of nests of Saunders's Gull in different nesting areas in the south of core area of Yancheng NNR, 1999–2007.

The overall mean turnover rate was 0.84 ± 0.08 (n = 7), whereas in Zhonglugang it was 0.85 ± 0.09 (n = 7) and in Sanlizha 0.79 ± 0.18 (n = 5) (Table 5). The turnover rates of nest sites differed between years for Sanlizha (t 6 = 24.936, P < 0.01) and Zhonglugang (t 6 = 3.612, P < 0.02). However, the overall turnover rate did not differ between years (t 6 = 1.695, P > 0.1).
Table 5. Turnover rates of nest-sites of Saunders's Gull in the south of core area of Yancheng NNR, 1999–2007.

The asterisk indicates that the turnover rate is 2000–2003 rather than 2000–2001.
Discussion
Breeding habitat changes in Yancheng coastal wetlands
Satellite images generally present the sole source of habitat data for many regions of the world important to wildlife (Gottschalk et al. Reference Gottschalk, Huettmann and Ehlers2005). The remote-sensing approach employed here to quantify changes in breeding habitats of Saunders's Gull indicates rapid and extensive declines in Common Seepweed with an annual loss in area of 5.27%. The majority of these changes can be attributed to the widespread expansion of aquaculture within the area. The number of patches of Common Seepweed has declined significantly in the past 15 years and the expansion of Smooth Cordgrass is likely to further fragment the remaining habitat. These changes seriously threaten the distribution of breeding sites of the ‘Vulnerable’ Saunders's Gull.
The alien Smooth Cordgrass was introduced into Yancheng coastal wetland in 1982 (Zhong et al. Reference Zhong, Zhou and Zhou1985). This plant developed from scattered patches in estuarine regions into a continuous belt mainly distributed on the periphery of coastal wetlands from Sheyang River to Liangduo River from 1992 to 2007 (Liu et al. Reference Liu, Zhang, Jiang and Wang2009a). Its spread has caused high levels of sediment accumulation, which constrains the natural transition of saline plants, especially Common Seepweed (Wang et al. Reference Wang, Gao and Jia2006). Considering that Saunders's Gull prefers to nest in areas with coverage of 20%–60% of Common Seepweed (Jiang et al. Reference Jiang, Chu, Qian and Lu2002), breeding sites will disappear if no management measures are taken to prevent expansion of Smooth Cordgrass in the short-term.
Although this alien plant has expanded extensively in the study area, the coastal wetland from Liangduo River to Xingang River has not been occupied by this plant and the area of Common Seepweed habitat is still increasing. This area was occupied by Saunders's Gull in 2007 (site code 9 in Figure 1) and is a potential breeding site for the gull if no land reclamation occurs in the future.
Threats to the Saunders's Gull population
Saunders's Gull is dependent upon salt marshes and tidal mudflats, both for breeding and for its specialised diet, which mainly comprises crabs, but also includes small fish, shellfish and Common Clamworm Perinereis kinberg (Brazil and Melville Reference Brazil and Melville1991, Brazil Reference Brazil1992). The main threats to this species include habitat loss and degradation, invasion of Smooth Cordgrass, direct and indirect human disturbance and cumulative predation risk.
Using a non-dense dimidiate pixel model of remote-sensed normalized difference vegetation index (NDVI) data, Liu et al. (2009b) concluded the mean Common Seepweed coverage of nesting sites of Saunders’s Gull was 34.98 ± 6.8% based on 184 nests in 2002 and 109 nests in 2007. Due to the expansion of Smooth Cordgrass and a lack of regular seawater flushing in the core area of Yancheng NNR, the mean coverage of Common Seepweed habitats increased from 27.6 ± 3.8% in 1992 to 35.4 ± 2.0% in 2002, and then to 53.1 ± 4.5% in 2007 (Liu et al 2009b). In this case, the suitable nesting habitats for Saunders’s Gull increased in 1992–2002, and then decreased 2002–2007.
In a stable and high quality breeding area, site fidelity can lead to higher reproductive success through the avoidance of predation and nest predation, improved feeding skills, and better adaptation to local circumstances in general (Vadász et al. Reference Vadász, Német, Karcza, Loránt, Biró and Csörgö2008). Saunders's Gull appeared to have a very high spatial turnover of nesting sites in the core area of Yancheng NNR and presumably also low site tenacity of individuals, which indicates that the gull is probably susceptible to disturbance in the same way as Lesser Black-backed Gull Larus fuscus (Virkkala Reference Virkkala2006).
Disturbance caused by human activities, such as collection of Common Seepweed, Common Clamworm and small crabs, has disrupted incubation and caused desertion of breeding sites. Other activities such as collection of Say's Paper-bubble Bullacta exarata, Onchidium struma and Lunatica gilva could disturb foraging birds and increase the risk of egg collection by local farmers. Meanwhile, fences for collection of small crabs will increase the risk of chicks falling into small jars placed along the fence (Jiang Reference Jiang2008). At present, the regulations in Yancheng NNR are lax and do not prevent continued building of new factories or restrict the increasing human activities.
Concluding remarks
Knowledge of population dynamics and habitat changes of Saunders's Gull can help development of management strategies for this ‘Vulnerable’ species. Preserving the breeding sites is a challenging task due to the species's spatio-temporal dynamics. New sites are continuously colonised and earlier inhabited sites become unoccupied. This is typical of colony-breeding gulls, such as Saunders's Gull, Black-headed Gull Larus ridibundus (Ulfvens Reference Ulfvens1993) and Lesser Black-backed Gull (Virkkala Reference Virkkala2006). Thus, it is recommended to conduct annual monitoring and to define a large enough range in one year to control disturbance to nesting, incubation and foraging, and increase breeding success.
In addition, habitat management is an important measure to prevent or reverse the direction of vegetation succession. Management of intertidal areas has also involved control of Common Reed and Smooth Cordgrass. It is recommended to use herbicide or mechanical disturbance to limit the expansion of Smooth Cordgrass, as has been applied in Europe to control Common Cordgrass Spartina anglica (Frid et al. Reference Frid, Chandrasekara and Davey1999). In addition, artificial intervention or burning to create suitable vegetation cover of Common Seepweed is also a critical aspect of habitat manipulation to provide suitable nesting areas for gulls.
Acknowledgements
We thank Yancheng National Nature Reserve for giving us permission to conduct this study. In particular, we would like to thank Dr. Liu Chunyue, Institute of Northeast Geography and Agriculture Ecology of Chinese Academy of Sciences who provided technical support on image interpretation and data processing, and Mr. Sun Guorong, Liu Xiaoyun and Liu Sanzai who were involved in the field work for several years. We appreciate the Department of Wildlife Conservation, State Forestry Administration of China, for funding various aspects of this research. We are grateful to Mr. Crawford Prentice of the International Crane Foundation for the revision and useful comments on an earlier draft of this manuscript. Two anonymous referees made useful comments on a previous version of the manuscript.















