Hepatic failure induced by the injection of d-galactosamine and lipopolysaccharide (GalN/LPS) has been considered as an inflammatory response, involving the accumulation of mononuclear cells in the liver and an increase in plasma alanine transaminase and aspartate aminotransferase activities. This phenomenon is observed in patients with acute hepatic failure( Reference Bélanger and Butterworth 1 ). GalN/LPS-induced liver injury is also known to cause cytokine release (TNF-α is the main mediator) that contributes to increased oxidative stress and the formation of reactive oxygen species, followed by hepatocyte death( Reference Oberholzer, Oberholzer and Bahjat 2 , Reference Liu, Zhang and Luo 3 ).
The blue-green alga Spirulina (Spirulina platensis, SP) is used as a health food source because it contains large amounts of vitamins, minerals and amino acids. Its consumption by humans and rodents is believed to be efficacious in improving diabetes( Reference Parikh, Mani and Iyer 4 ), osteopenia( Reference Ishimi, Sugiyama and Ezaki 5 ) and immunity( Reference Hirahashi, Matsumoto and Hazeki 6 ). Furthermore, it has been reported that SP and its component phycocyanin mitigate d-galactosamine-induced liver injury in rodents( Reference Lu, Ren and Wang 7 , Reference González, González and Remirez 8 ). Recently, studies have reported that not only SP but also Fe- and Se-rich SP are beneficial to human health( Reference Puyfoulhoux, Rouanet and Besançon 9 – Reference Cases, Wysocka and Caporiccio 11 ).
It has been reported that some metals such as zinc and gallium reduce liver damage( Reference Zhou, Wang and Song 12 , Reference Krecic-Shepard, Shepard and Mullet 13 ), and germanium is also considered to exhibit this effect. Germanium is present in all living plant and animal matter in micro-trace quantities. Although inorganic germanium (germanium dioxide) has been shown to have toxic effects( Reference Lin, Chen and Chen 14 ), organic germanium has therapeutic attributes including immune enhancement( Reference Nakamura, Saito and Aso 15 ) and antioxidative effects( Reference Goodman 16 , Reference Alil, Noaman and Kamal 17 ). Organic germanium is well known to be effective in protecting against liver injury in mice through reducing the production of interferon-γ (IFN-γ) or TNF-α( Reference Yokochi, Ishiwata and Hashimoto 18 , Reference Ishiwata, Yokochi and Hashimoto 19 ). Thus, germanium-containing Spirulina (GeSP) could be expected to show stronger hepatoprotective activity than SP due to the synergistic effects of both germanium and SP. Therefore, in the present study, we produced GeSP and examined its effect.
The safety of GeSP, which is cultured in the presence of germanium dioxide, has been confirmed in animals( Reference Orimo, Onda and Kaseki 20 ). There were almost no differences between the GeSP-fed group and the control groups in the growth, weight and histological findings of the organs (e.g. brain, heart, liver and kidney).
In the present study, the protective effects of dietary SP and GeSP were compared in rats with GalN/LPS-induced liver injury by measuring parameters related to liver injury.
Materials and methods
Spirulina germanium fortification
SP was grown in a basal medium (pH 10·5) supplied with aeration. All chemicals were purchased from Wako Pure Chemical Industries. The basal medium contained Na2CO3 (12·6 g/l), NaHCO3 (10·0 g/l), Na2SO4 (3·0 g/l), NaNO3 (2·2 g/l), KCl (0·5 g/l), K2HPO4 (0·5 g/l), MgCl2.6H2O (0·5 g/l), EDTA (0·07 g/l), CaCl2.2H2O (0·05 g/l), FeSO4.7H2O (5·0 mg/l), H3BO3 (286 μg/l), MnCl2.4H2O (184 μg/l), ZnSO4.7H2O (220 μg/l), CuSO4.5H2O (19·7 μg/l), KCr(SO4)2.12H2O (48·0 μg/l), MoO3 (37·5 μg/l), NH4VO3 (23·0 μg/l), NiSO4.6H2O (22·5 μg/l), Co(NO3)2.6H2O (24·5 μg/l), Ti(SO4)2 (4·0 μg/l) and Na2WO2.2H2O (1·8 μg/l), and was adjusted to pH 10·5. GeSP was grown in this medium in the presence of germanium dioxide. The pH was adjusted to 12 by adding 5 % NaOH. At the end of the growth period, biomass was recovered and filtered through a 20 μm membrane, thoroughly washed with tap water and dried.
Animal care
The care and use of the rats followed the institutional guidelines of Yamagata University. Male Wistar rats (6 weeks old) were purchased from Japan SLC, Inc. The rats were housed individually in stainless-steel cages with a 12 h light–12 h dark cycle at 22 ± 2°C and 40–60 % humidity. Diet and water were given ad libitum. After acclimatisation for 5 d, the rats were divided into five groups based on the body weight of the rats. Statistics were used to ensure that there were no significant differences between the groups. Of these five groups, two were fed on a basal diet (CON and GalN/LPS-CON; n 6) and three were fed on a basal diet supplemented with 5 % SP or GeSP (GalN/LPS-SP and GalN/LPS-GeSP; n 7) (Table 1). As SP and GeSP contained about 60 % protein, the quantity of casein was adjusted in such a way that the protein level was equal among all the diets. The diet that included GeSP contained 30 parts per million germanium. The concentration of germanium was determined by flame atomic absorption spectrometry.
Table 1 Composition of the diets (%)
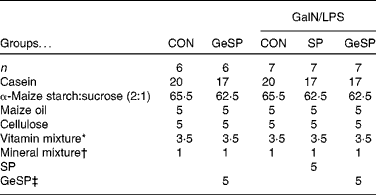
GalN/LPS, d-galactosamine and lipopolysaccharide; CON, group fed on basal diet; GeSP, germanium-containing Spirulina; SP, Spirulina.
* AIN-93G-MX, which contained 25 g bitartrate/100 g, were obtained from Oriental Yeast Company Limited.
† AIN-93-VX, which contained 25 g bitartrate/100 g, were obtained from Oriental Yeast Company Limited.
‡ The diet that included GeSP contained 30 parts per million germanium.
After administering these diets for 7 d, rats assigned to the GalN/LPS groups were intraperitoneally injected with GalN/LPS (800 mg GalN+30 μg LPS/kg body weight, GalN (d-galactosamine hydrochloride) and LPS (from Escherichia coli)). Rats in the other groups were injected with saline. At 22 h after the injection of GalN/LPS, rats from each group were anaesthetised and blood was collected by cardiac puncture. Plasma was prepared by centrifuging the heparinised whole blood at 1000 g for 20 min. A portion of the liver was excised, quickly frozen in liquid N2 and stored at − 80°C until use.
Measurement of plasma enzyme activities and cytokine levels
The activities of alanine transaminase, aspartate aminotransferase and lactate dehydrogenase were measured enzymatically with a commercial kit, i.e. the Transaminase CII test and LDH-UV test, respectively (Wako Pure Chemicals).
IFN-γ, IL-10 and TNF-α levels were also measured by using commercial ELISA kits, i.e. the Amersham Interferon Gamma Rat Biotrak ELISA System, the Amersham Interleukin-10 Rat Biotrak ELISA System and the Amersham Tumor necrosis factor Alpha Rat Biotrak ELISA System, respectively (GE Healthcare).
Measurement of liver antioxidant enzyme activities
Liver samples (0·6 g) were homogenised with a 5-fold volume of potassium phosphate buffer (0·1 m, pH 7·4, containing 1 mm-EDTA) and a 2-fold volume of KCl (2·3 %), followed by centrifugation at 10 000 g for 20 min at 4°C. The supernatants obtained were used for the measurement of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activities.
Superoxide dismutase activity was measured by using the xanthine/xanthine oxidase system( Reference Imanari, Hirota and Hayakawa 21 ). Catalase activity was determined by using the method of Chance & Meahly( Reference Chance and Meahly 22 ), i.e. measuring the decrease in absorbance at 240 nm due to the decomposition of H2O2. Glutathione peroxidase activity was determined from the decrease in the NADPH level due to the reaction( Reference Paglia and Valentine 23 , Reference Whanger, Weswing and Schmitz 24 ). Glutathione reductase activity was also measured by monitoring the decrease in the absorbance of NADPH at 340 nm by using oxidised glutathione as a substrate( Reference He and Yasumoto 25 ). Protein concentrations were determined by using the method of Lowry et al. ( Reference Lowry, Rosebrough and Farr 26 ), using bovine serum albumin as the standard.
Measurement of the levels of glutathione, thiobarbituric acid-reactive substances, α-tocopherol and ascorbic acid
To measure the levels of the reduced form of glutathione and the oxidised form of glutathione (GSSG), homogenates were prepared by homogenising liver samples with a 10-fold volume of 0·036 M-perchloric acid. The supernatant obtained by centrifuging the homogenates at 13 500 g for 5 min at 4°C was filtered through a 0·2 μm filter. Reduced form of glutathione and GSSG contents in the filtrate were determined by ion-pairing reverse-phase HPLC coupled to a coulometric detector according to the method of Harvey et al. ( Reference Harvey, Ilson and Strasberg 27 ).
The concentration of TBARS was measured according to the method of Uchiyama & Mihara( Reference Uchiyama and Mihara 28 ), using a homogenate prepared by mixing 0·5 g of the frozen liver sample with a 9-fold volume of a cold solution of 1·15 % KCl.
Liver α-tocopherol and ascorbic acid levels were determined by using HPLC, according to the method of Sugimoto et al. ( Reference Sugimoto, Igarash and Takenaka 29 ).
Statistical analyses
The normality of the animal distribution was tested on the body weight of the rats using a one-way ANOVA with Tukey's test. Results were analysed using Student's t test if two groups (CON and GeSP groups) were compared or by a two-way ANOVA followed by Tukey's test if three groups (among the GalN/LPS groups) were tested. If variances were inhomogeneous in Student's t test, results were analysed using the Welsh test. Data are expressed as means with their standard errors. A P value of less than or equal to 0·05 was considered as significant.
Results and discussion
The protective effects of SP and GeSP against GalN/LPS-induced liver injury in rats are given in Table 2. No differences were observed in body-weight gain and food intake among the groups, regardless of the treatment with GalN/LPS. Plasma alanine transaminase, aspartate aminotransferase and lactate dehydrogenase activities were significantly lower in the GalN/LPS-GeSP group than in the GalN/LPS-CON group. Although the alanine transaminase activity in the GalN/LPS-SP group had a tendency to decrease (P< 0·1), the aspartate aminotransferase activity was not different from that in the GalN/LPS-CON group. Lactate dehydrogenase activities in the GalN/LPS-SP and GalN/LPS-GeSP groups were significantly lower than that in the GalN/LPS-CON group; however, there was no difference between the GalN/LPS-SP and GalN/LPS-GeSP groups. Although a histological evaluation of the liver was not performed in the present study, previous studies have shown that there were no histopathological differences in the liver of rats fed SP or GeSP when compared with rats fed a normal diet( Reference Orimo, Onda and Kaseki 20 , Reference Yoshino, Hirai and Takahashi 30 ). With regard to parameters related to liver injury, the present results suggest that the liver of rats in the GalN/LPS-SP and GalN/LPS-GeSP groups was not as damaged as the liver of rats in the GalN/LPS-CON group. In particular, the results for aminotransferase activities indicate that liver damage in rats fed the GalN/LPS-GeSP diet was milder than that in rats fed the GalN/LPS-SP diet.
Table 2 Effects of dietary Spirulina (SP) and germanium-containing Spirulina (GeSP) on d-galactosamine- and lipopolysaccharide-induced hepatitis in rats (Mean values with their standard errors)

GalN/LPS, d-galactosamine and lipopolysaccharide; CON, group fed on basal diet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; IFN-γ, interferon-γ; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GSSG-R, glutathione reductase; GSH, reduced form of glutathione; GSSG, oxidised form of glutathione; TBARS, thiobarbituric acid-reactive substances.
Mean values were significantly different from those of the GalN/LPS-CON group: † P< 0·1, * P< 0·05, ** P< 0·01.
†† Mean values were significantly different from those of the CON group (P< 0·1).
Feeding of GeSP significantly inhibited the GalN/LPS-mediated increase in plasma IFN-γ and TNF-α levels. The levels of these cytokines tended to be lower in the GalN/LPS-SP group than in the GalN/LPS-CON group (P< 0·1). Moreover, plasma levels of IL-10 in the GalN/LPS-SP and GalN/LPS-GeSP groups were significantly elevated compared with those in the GalN/LPS-CON group. The animals injected with GalN/LPS have been reported to develop severe liver injury, which is dependent on the induction of TNF-α and IFN-γ( Reference Josephs, Bahjat and Fukuzuka 31 , Reference Kim, Hong and Radaeva 32 ), whereas IL-10 protects from liver injury through reducing the mRNA expression of IFN-γ and TNF-α( Reference Nagaki, Tanaka and Sugiyama 33 ). These results support the possibility that the administration of SP and GeSP protects against liver damage induced by the injection of GalN/LPS; furthermore, the results show that GeSP was more effective in alleviating liver damage than SP.
GalN/LPS-induced liver injury is also known to cause increased formation of reactive oxygen species, followed by hepatocyte death( Reference Oberholzer, Oberholzer and Bahjat 2 , Reference Liu, Zhang and Luo 3 ). Therefore, we analysed the activities of liver antioxidant enzymes and glutathione levels. Superoxide dismutase and catalase activities did not differ between the CON and GeSP groups that received no GalN/LPS treatment. The increased catalase and glutathione peroxidase activities observed following the injection of GalN/LPS were decreased in the group fed on GeSP. Although GSSG levels did not differ between the two groups that were not injected with GalN/LPS, the GSSG level in the GalN/LPS-GeSP group was significantly lower than that in the GalN/LPS-CON group. It has been reported that GSSG levels are increased by oxidative stress( Reference Sugimoto, Igarash and Takenaka 29 ). These results may suggest that SP, especially GeSP, ameliorates oxidative stress induced by the injection of GalN/LPS. However, GeSP tended to reduce the activities of antioxidant enzymes (glutathione peroxidase and glutathione reductase) in the absence of GalN/LPS treatment. At present, the mechanism by which GeSP influences these enzymes is unclear. Goodman( Reference Goodman 16 ) suggested that organic germanium (bis(carboxylgermanium) sesquioxide) might function as an electron donor. Thus, GeSP itself may also act as an antioxidant in the body. A study has reported that high amounts of phycocyanobilin present in Spirulina inhibit the activity of NADPH oxidase( Reference Ravi, De Lata and Azharuddin 34 ). Moreover, various studies have suggested that germanium compounds may have protective effects against liver injury, as well as antioxidant effects( Reference Alil, Noaman and Kamal 17 , Reference Ishiwata, Yokochi and Hashimoto 19 , Reference Orimo, Onda and Kaseki 20 ). Thus, GeSP is believed to exert synergistic effects of both Spirulina and germanium.
Furthermore, liver α-tocopherol, ascorbic acid and TBARS levels were measured to determine the status of oxidative stress in the liver. There were no differences in the levels of α-tocopherol and ascorbic acid in the CON and GeSP groups that were not injected with GalN/LPS. However, their levels decreased in rats injected with GalN/LPS and the levels improved in those fed with GeSP, but not with SP. These (α-tocopherol and ascorbic acid) might not be consumed, because Gesp itself functions as an antioxidant in the body. The level of TBARS in the GalN/LPS-GeSP group had the tendency to decline compared with that in the GalN/LPS-CON group (P= 0·102), but there was no significant difference between the groups. These results suggest that the high oxidative status of the rat liver following the injection of GalN/LPS was improved by the intake of GeSP. However, the intake period employed in the present study might be too short to have an effect on the lipid peroxide level.
The present study suggests that the intake of GeSP affects the activities of antioxidant enzymes and reduces oxidative stress. Furthermore, it indicates that dietary GeSP exerts a stronger protective effect than SP against liver injury induced by the injection of GalN/LPS. Thus, dietary GeSP could provide beneficial effects to human health.
Acknowledgements
The present study was supported by a research grant from Ryusendo Company Limited, Japan. O. Y. designed and conducted the research, analysed the data and wrote the paper. Y. S. and K. I. conducted and directed the research. All authors read and approved the final manuscript. There are no conflicts of interest to disclose.






