Introduction
The rising resistance to antimicrobials observed among an increasing number of microorganisms represents a direct threat to human health. Patients infected with a resistant microorganism are less likely to recover with the first antimicrobial therapy and are likely to require extra investigation and treatment – and a number of antimicrobial drugs may be needed to eradicate the infection (Cosgrove, Reference Cosgrove2006). This, in turn, results in longer hospital stays, higher morbidity and mortality for patients, as well as higher costs for the health care sector and society as a whole.
This chapter provides an overview of the health and economic burden of antimicrobial resistance (AMR). It first presents the current state of knowledge on the epidemiology of AMR and discusses the main analytical challenges in determining the current and long-term effects of resistance on populations in terms of morbidity, mortality, and length of hospital stays. In addition, a summary of the current literature on the economic impact of AMR is provided along with a detailed discussion of the characteristics and limitations of existing economic models. Finally, it identifies the main knowledge gaps and suggests avenues for future research and approaches to address them.
The effect of AMR on morbidity and mortality
AMR has profound consequences for the treatment of infections. This limitation of treatment options often makes it necessary to resort to antibiotics with a broader spectrum of action, some of which are potentially less effective or safe than narrow-spectrum antibiotics. Resistance also affects empirical treatment – where the clinician selects an antibiotic for the treatment of an infection in the absence of microbiological results – which might result in an underestimation of the risk associated with specific infections and the use of inappropriate antibiotics. For example, results from a meta-analysis showed that patients with bacteraemia caused by resistant Enterobacteriaceae are five times more likely to experience delays in receiving an effective therapy compared to patients infected by a susceptible strain (Schwaber & Carmeli, Reference Schwaber and Carmeli2007). This may impair the long-term effectiveness of antibiotics, delay access to effective treatments, increase the rates of treatment failure with concomitant complications, and eventually lead to higher fatality rates. Outcome studies have consistently demonstrated increased length of stay (LOS) in hospital, greater need for surgery, and higher mortality for infections caused by resistant Gram-positive and Gram-negative bacteria (Lambert et al., Reference Lambert, Suetens and Savey2011). Figure 2.1 illustrates the higher mortality associated with resistant infections for selected bug–drug combinations.

Figure 2.1 Relative risk of 30-day mortality of patients with resistant infections relative to those with susceptible infections
Notes: 3CRKP: Third-generation cephalosporin-resistant Klebsiella pneumoniae; CRKP: Carbapenem-resistant Klebsiella pneumoniae; MRSA: Methicillin-resistant Staphylococcus aureus; FREC: Fluoroquinolone-resistant Escherichia coli; 3CREC: Third-generation cephalosporin-resistant Escherichia coli.
Another study calculated the health burden of selected antibiotic- resistant bacteria of public health importance in European Union/ European Economic Area (EU/EEA) countries expressed in disabilityadjusted life-years (DALYs) (Cassini et al., Reference Cassini, Högberg and Plauchouras2018a). Their model was populated with estimated incidence stemming from data reported to the European Antimicrobial Resistance Surveillance Network (EARS- Net) and the European Centre for Disease Prevention and Control (ECDC) point prevalence survey of health care-associated infections and antimicrobial use in European acute care hospitals in 2011–2012 (ECDC, 2013). Moreover, data retrieved from systematic reviews of published literature provided evidence on the attributable case fatality and attributable length of stay for selected infections with antibiotic-resistant bacteria. Results showed that an estimated 671 689 (95% uncertainty intervals (UI) 583 148–763 966) infections occurred in 2015 in EU/EEA countries, accounting for 33 110 (95% UI 28 480–38 430) attributable deaths and 874 541 (768 837–989 068) DALYs. The burden of infections with bacteria resistant to antibiotics on the EU/ EEA population was comparable to that of influenza, tuberculosis and HIV/AIDS combined (Cassini et al., Reference Cassini, Colzani and Pini2018b) and, between 2007 and 2015, the burden of each of the 16 antibiotic-resistant bacteria under study has increased. The study also showed that 75% of the burden measured in DALYs was due to health care-associated infections and that this could be minimized through adequate infection prevention and control (IPC) measures, as well as antibiotic stewardship in health care settings. Finally, the contribution of various antibiotic-resistant bacteria to the overall burden varied greatly between countries, thus prevention and control strategies should be tailored to the needs of each individual country.
The impact of AMR is more serious in hospitalized patients and particularly for vulnerable groups such as immunocompromized patients (e.g. those with cancers receiving chemotherapy, having undergone organ transplantation or receiving immunosuppressive treatment) and the critically ill. These patients are also exposed to a higher risk of colonization by resistant bacteria through contact with health care delivery services, the invasive procedures their conditions often require, and frequent antibiotic treatments.
AMR also threatens the effectiveness of antibiotics as a prophylactic measure, leading to the use of broader-spectrum drugs and increasing the selection pressure for the emergence and spread of resistant strains, thereby further exacerbating the spread of AMR. Such a sequence of events jeopardizes the performance and safety of many common surgical procedures and cancer treatments that rely on effective antibiotic prophylaxis. A recent study investigated the potential health consequences of increases in antibiotic resistance for the 10 most common surgical procedures and immunosuppressing cancer chemotherapies that rely on antibiotic prophylaxis in the United States (Teillant et al., Reference Teillant, Gandra and Barter2015). Their model showed that a 30% reduction in the efficacy of antibiotic prophylaxis for the included procedures would result on average in 120 000 additional surgical site infections and infections after chemotherapy per year (ranging from 40 000 for a 10% reduction in efficacy to 280 000 for a 70% reduction in efficacy), and 6 300 infection-related deaths (ranging from 2 100 for a 10% reduction in efficacy, to 15 000 for a 70% reduction).
The effect of AMR on the incidence of infections: replacement or addition?
In addition to the consequences on treatment effectiveness, morbidity, and mortality, AMR can also affect the number of infections in two possible ways. First, infections by resistant microorganisms can replace infections by susceptible (i.e. non-resistant) organisms through ecological replacement. In this scenario, the total number of infections remains stable as the number of infections by susceptible organisms decreases, resulting overall in increased morbidity due to resistance. Second, it has been hypothesized that the effect of some infections by resistant microorganisms may be additive to the burden of the same infections by susceptible microorganisms. In this case, the total number of infections would increase and the added burden would be the result not only of resistance but also of the additional infections. The majority of studies on methicillin-resistant Staphylococcus aureus (MRSA) suggest this possibility. MRSA predominantly adds to the burden of infections by methicillin-susceptible Staphylococcus aureus, as the incidence of infection by the latter has not decreased either in the hospital or the community, despite the increase of MRSA infections (Mostofsky, Lipsitch & Regev-Yochay, Reference Mostofsky, Lipsitch and Regev-Yochay2011). However, a combination of additive and replacement effects cannot be excluded. Additional factors may also play a role in the respective changes in incidence of infections by resistant and susceptible microorganisms. Such factors include possible differences in virulence between susceptible and resistant strains, as was shown for Panton–Valentine leucocidin-producing community-associated MRSA (Martinez-Aguilar et al., Reference Martinez-Aguilar and Hulten2004). It is also likely that the predominance of additive or replacement effects differs among species or even strains of the same species.
Challenges in estimating the health burden of resistance
In a narrow sense, AMR refers to the phenomenon of a microorganism being resistant to the effect of a particular antimicrobial. It can be an intrinsic property of a microbial species (intrinsic resistance) or acquired by some members of the species (acquired resistance) through genetic or translational modifications. There is often cross-resistance to antimicrobials of the same class. For example, resistance to carbapenems implies resistance to all, or almost all, other beta-lactams. Even more alarming is the increasing frequency of microorganisms that are resistant to multiple antimicrobial classes. For example, in EU/EEA countries in 2016, 15.8% of Klebsiella pneumoniae isolates, on average, were resistant to beta-lactams, fluoroquinolones and aminoglycosides (ECDC, 2016).
There are a multitude of factors that determine the burden of resistance of a given pathogenic microorganism to a particular antimicrobial, including the site and severity of infections, the intrinsic resistance of particular species, and the available alternative antimicrobials that can potentially be used for the treatment of infections caused by resistant strains. In the worst-case scenario, a microorganism can be resistant to most or even all available antimicrobials, making treatment extremely challenging.
The effects of AMR are complicated by the fact that manifestations, complications, and treatment outcomes are specific to each patient. Ideally, estimation of the burden of resistance would consist of estimates for each particular microorganism and each specific antibiotic in different hosts, while at the same time accounting for multidrug-resistance phenomena. Due to the enormity of such a task, estimations of the burden of AMR currently focus on specific sites of infections, microorganisms and antimicrobial combinations.
Disease selection
From an epidemiological point of view, the selection of the pathogens and resistance combinations to assess presents an important challenge in estimating the health burden of AMR. It is also a critical step as the included pathogens and resistances will be used as indicators to determine and monitor AMR and will have a significant impact on outcome and forecasting studies, as well as on the evaluation of AMR prevention strategies.
Most efforts to estimate the burden of resistance so far have focused on specific bug–drug combinations that are common and considered clinically important due to the severity of the infections they cause and/ or the limited number of treatment options available. These combinations include: MRSA, vancomycin-resistant enterococci (VRE), extended spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii, drug-resistant Neisseria gonorrhoeae and Streptococcus pneumoniae.
For example, in 2013 the US Centers for Disease Control and Prevention (CDC) provided information on the number of cases and deaths due to a selection of pathogens resistant to a number of antimicrobials (CDC, 2013). More than 2 million infections were estimated to have caused at least 23 000 deaths. In terms of mortality, the highest burden was associated with infections due to CRE, multidrug-resistant Acinetobacter, ESBL-producing Enterobacteriaceae, VRE, multidrug-resistant Pseudomonas aeruginosa, MRSA, and Streptococcus pneumoniae. Other infections such as drug-resistant Candida, drug resistant Neisseria gonorrhoeae, or drug-resistant Campylobacter are important to survey in order to follow their resistance evolution, but do not seem to cause a significant number of deaths.
In Europe in 2009, the European Centre for Disease Prevention and Control (ECDC) estimated that 386 100 resistant infections accounted for 25 100 deaths and more than 2.5 million extra hospital days (Table 2.1). MRSA had the highest incidence among AMR infections in the European context, and it was estimated that carbapenem-resistant Pseudomonas aeruginosa caused the highest number of deaths (ECDC/EMA, 2009).
Table 2.1 Estimated yearly human burden of infections due to the selected antibiotic-resistant bacteria in EU Member States, Iceland and Norway in 2007
| No. cases of infections | No. extra deaths | No. extra hospital days | |
|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus (MRSA) | 171 200 | 5 400 | 1 000 |
| Vancomycin-resistant Enterococcus faecium | 18 100 | 1 500 | 111 000 |
| Penicillin-resistant Streptococcus pneumoniae | 3 500 | N/A | N/A |
| Third-generation cephalosporin- resistant Escherichia coli | 32 500 | 5 100 | 358 000 |
| Third-generation cephalosporin- resistant Klebsiella pneumoniae | 18 900 | 2 900 | 208 000 |
| Carbapenem-resistant Pseudomonas aeruginosa | 141 900 | 10 200 | 809 000 |
| Total | 386 100 | 25 100 | 2 536 000 |
Note: N/A: Not available.
Data availability and sources
Estimating the incidence of AMR is challenging as the scope of most surveillance systems and data sources is to determine the proportion of resistant pathogens over the total amount of tested infections.
However, the number of tested samples is heterogeneous across countries and is based on a number of factors ranging from laboratory capacity, and frequency of microbiological testing to health care system organization.
Another relevant element to consider when assessing data sources is the purpose of the surveillance system or study in question. Screening, as opposed to syndromic testing, provides information on the number of positive carriers of a resistant microorganism, not necessarily suffering from an infection. For example, the ECDC’s EARS-Net, similar to the Central Asian and Eastern European Surveillance of Antimicrobial Resistance (CAESAR) coordinated by the European Regional Office of the WHO, collects data only from blood and cerebrospinal fluid (CSF) for a selected number of pathogens and provides information on their susceptibility, intermediate or resistance status. The Pan American Health Organization Red Latinoamericana de Vigilancia de la Resistencia Antimicrobiana (ReLAVRA) also provide information on the proportion of resistant infections, although it is not limited to blood and CSF. For these two registries, however, differences in data quality and number of samples undermine comparability at the regional level. Finally, the recent ambitious Global Antimicrobial Resistance Surveillance System (GLASS) initiative from the WHO aims to provide information on the incidence of susceptible and resistant infections. However, the GLASS experience is too recent to allow a critical assessment of the quality of the data and it is likely that it will take time before optimal quality standards are reached globally (WHO, 2018). In a number of countries, such as Sweden, Greece, and the United Kingdom, mandatory notification of resistant bloodstream infections by particular resistant microorganisms is in place (e.g. MRSA, VRE, colistin and carbapenem resistance).
In the absence of widely available incidence data for most of the infections caused by resistant microorganisms, modelling provides another approach to estimate incidence from the available data on the proportions of resistance.
Choice of comparator
The choice of the comparator used for the estimation of the attributable case fatality/mortality and attributable LOS in hospital has an impact on the usability of results for assessing interventions. When comparing outcomes of resistant against susceptible infections, the difference measured is between treatment options for the same pathogen. On the other hand, when the comparator is no infection, the resistant infection is treated as any other infection caused by a specific pathogen (the resistant pathogen is considered distinct from the susceptible version of the same pathogen). Interventions aim at preventing AMR focus on infection control in hospitals (e.g. hand hygiene) and antibiotic stewardship. Most health care-associated infection control strategies will have an effect on all infections, irrespective of their resistance pattern. Therefore, valuable information is provided by studies on the burden of AMR using a non-infected population as comparator.
On the other hand, antibiotic stewardship aims mainly at preventing the emergence of resistance. Hence, studies describing the added burden of disease caused by resistance as opposed to that of the susceptible infections inform the effectiveness of the antibiotic stewardship intervention under investigation.
Trends in resistance rates
Although there is a general trend of increasing resistance to available antimicrobials, this trend is not uniform across European countries for bacterial species and antimicrobials. For example, EARS-Net data show decreasing rates of MRSA in several European countries (ECDC, 2017). This change has been particularly significant in countries where a national plan on infection control and antimicrobial stewardship was implemented.
By contrast, an alarming increase in resistance rates is observed in Gram-negative bacteria and especially Enterobacteriaceae. Multidrug-resistant Escherichia coli and Klebsiella pneumoniae have been increasing over the last five years. For example, EU/EEA rates of E. coli resistant to third-generation cephalosporins increased from 8.2% in 2009 to 13.1% in 2015. These infections are predominant in the community and are generally associated with a high and inappropriate use of antibiotics in primary care. This indicates the need to improve stewardship programmes for general practitioners.
Resistance to carbapenems, a group of antibiotics used to treat severe health care-associated infections caused by multidrug-resistant bacteria, has been spreading globally. It has led to an increase in consumption of polymyxins in several countries and, in turn, to the emergence and spread of polymyxin-resistant Enterobacteriaceae (Grundmann et al., Reference Grundmann, Glasner and Albiger2017). Resistance to polymyxins, which are last-resort antimicrobials, seriously limits the treatment options for such infections. This serves as a sign of both overuse and misuse of antibiotics in hospitals, as well as poor IPC.
AMR as a negative externality in the health care sector and beyond
In economic terminology, AMR is an externality (i.e. an activity causing an effect on third parties) resulting from the use of antimicrobials to treat infections. This means that the effect of antimicrobial use in a particular patient, in terms of selection pressure and subsequent drug resistance, may not initially be felt directly by the patient or the clinician but will ultimately impact the overall welfare of other patients in the community and have adverse social and economic effects (Coast, Smith & Millar, 1996). Determining the cost of resistance is therefore a complex task that cannot be easily performed.
The first challenge in assessing the economic burden of AMR comes from the fact that its cost is partly hidden – as neither the immediate consumer, nor the supplier of the antimicrobial, has to bear the full cost of inappropriate usage. The level of complexity increases as, similar to the health burden, estimating the economic burden requires taking into account the specificity of each microorganism in terms of single or combined resistance, treatment procedures, and associated costs.
A second challenge comes from the fact that AMR compromises the success of many medical interventions that depend on the effective treatment and prevention of infection; for example: immunosuppressive therapies, chemotherapies and surgeries. Thus, AMR can undermine the safety of hospitals and that of many interventions that require antimicrobial prophylaxis. Therefore, determining the full economic impact of resistance on health care systems requires:
1) a better understanding of the epidemiology of resistance in the context of antimicrobial prophylaxis and iatrogenic infection prevention strategies;
2) identification and measurement of the costs induced by resistance for each individual procedure.
The third challenge is that the effect of AMR goes beyond public health and has potential detrimental impacts on a number of social and economic sectors (e.g. the labour market, livestock industries, the tourism industry). Assessing the economic burden of AMR implies that its associated costs, across various sectors of the economy, should be clearly identified and measured.
Impact of AMR on the health care budget
Additional health care costs due to AMR are driven by a variety of factors such as prescription of ineffective antibiotics, delayed initiation of antimicrobial therapies, and the severity of resistant infections and the additional care they require. The treatment cost of a resistant infection has been estimated to be between $10 000 and $40 000 higher than that of a susceptible infection (Sipahi, Reference Sipahi2008; Cohen et al., Reference Cohen, Larson and Stone2010; Smith & Coast, Reference Smith and Coast2013; Tansarli et al., Reference Tansarli, Karageorgopoulos and Kapaskelis2013; WHO, 2014). A recent modelling study conducted by the Organisation for Economic Co-operation and Development (OECD) including 33 EU and OECD countries estimated the extra health care expenditure due to AMR at around $3.5 billion per year. By 2050, the cumulative cost of AMR to the health care system of those countries is expected to reach $134 billion (OECD, 2018). The main drivers underlying this extra cost include:
the use of second-line antibiotics (which are usually more expensive), or application of different combinations of antibiotics before identifying the most effective strategy;
advanced laboratory tests to identify effective therapies for specific agents or imaging to monitor the development of complications associated with a given resistant infection;
higher treatment intensity including hospitalization in the case of resistant community-acquired infections. If a patient develops a resistant infection during hospitalization, transfer to intensive care and isolation measures will substantially increase treatment cost;
higher probabilities of undergoing surgical procedures for patients with resistant infections; these procedures may range from removal of infected tissue to amputation (Cosgrove, Reference Cosgrove2006);
excess LOS or treatment until the infection is eradicated. This entails use of additional medical and hospital resources;
changes in physicians’ prescribing habits as they may start prescribing second-line antibiotics to treat first-line antibiotic susceptible infections if the prevalence of resistance is perceived as high (McNulty et al., Reference McNulty, Lasseter and Charlett2011).
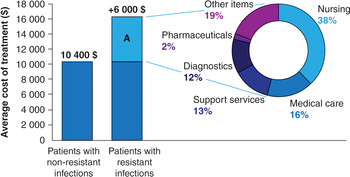
A recent study calculated the contribution of the different items to the total health care expenditure of patients with an E. coli bloodstream infection (Tumbarello et al., Reference Tumbarello, De Pascale and Trecarichi2013) (Figure 2.2). More than half of the extra expenditure was allocated to costs associated with additional nursing and medical care, while pharmacy services (e.g. second-line therapies, broad-spectrum drugs, disposables) accounted for less than 2% of the additional costs. In some cases, the contribution of the pharmacy services, particularly in terms of second-line therapies, to the additional expenditure associated with AMR may become much larger than the estimate reported, both in absolute and relative terms. Filice and colleagues (2010) found that the costs of antibiotics to treat resistant strains of S. aureus were on average seven times higher than the cost of treating susceptible infections – $142 as opposed to $21.

Figure 2.2 Cost of hospitalization for patients with Escherichia coli antibiotic-resistant infection and underlying drivers.
Note: Section A reflects the additional average costs for those patients hospitalized with E. coli-resistant infections.
In the United States, the cost associated with treating ear infections increased by 20% (equivalent to $216 million) between 1997 and 1998 due to resistance (Sharma & Towse, Reference Sharma and Towse2011). The WHO estimated that the cost of treating multidrug-resistant tuberculosis in high-income countries can range from $35 000 to $41 000 per case (Fitzpatrick & Floyd, Reference Fitzpatrick and Floyd2012). But this cost may become much higher in the case of extensively drug-resistant tuberculosis. Several reports have documented cases with treatment costs exceeding $200 000 and at least one case with a total cost close to $1 million (Chaulk & Kazandjian, Reference Chaulk and Kazandjian1998).
Long-term societal costs of AMR
The effect of AMR on health budgets is substantial but represents only a small fraction of the potential financial and human consequences of resistance on society. The effects of AMR on societal outcomes are determined mainly by the higher morbidity and mortality it leads to. These two factors affect the size of the labour force and labour productivity. More specifically, AMR is associated with societal costs resulting from lost income due to longer time away from work, the costs associated with ill-health and, eventually, death.
A study calculated the costs attributable to mortality and productivity loss during the extended time spent in hospitals for a cohort of US patients in 2000 and estimated the societal costs associated with resistant infections at around $38 000 per patient – more than double the medical costs (Roberts et al. Reference Roberts, Hota and Ahmad2009). This estimate does not include other potential costs incurred by the families of hospitalized persons (e.g. travel time or absence from work to care for the patient). The authors estimated that scaling up these figures to the national level would mean that the US population, in 2000, had lost about $35 billion (or about 0.35% of the national GDP) due to lost wages and premature deaths. This figure does not account for antimicrobial-resistant infections in the community. Similarly, it was calculated that, in Europe, productivity losses due to absence from work caused by AMR amounted to about €600 million in 2007 (ECDC/EMA, 2009).
At the population level, it was estimated that by 2020, the working-age population in OECD countries could be 0.6 million lower than its level in 2014 due to AMR (Taylor et al., Reference Taylor, Hafner and Yerushalmi2014). By 2050, the total loss in people within productive age could rise to 2.1 million. Both estimates assume no increase in the level of resistance, which is an unlikely scenario, particularly if no significant action is taken against AMR. Figure 2.3 presents projections to 2050 of the potential effect of AMR on the labour force. Under two hypothetical scenarios of resistance rates of 40% and 100%, the model predicted that by 2050 the total annual number of deaths in the working-age population would reach, 4 and 10.2 million, respectively.

Figure 2.3 Projected working-age population loss in OECD countries per year relative to 0% resistance, 2020–2050.
Macroeconomic effects of AMR
The macroeconomic effects of AMR are likely to be significant and to affect a number of sectors. As mentioned in the previous section, AMR has a detrimental effect on the labour force participation and productivity as well as on the size of the population. Both of these factors are key drivers of economic growth (Bloom, Canning & Sevilla, Reference Bloom, Canning and Sevilla2004) and several studies have attempted to provide global estimates of the economic burden of AMR by taking them into account.
In 2014, KPMG analysed the global economic impact of AMR and its potential evolution by 2050 (KPMG, 2014). The study assessed four alternative resistance scenarios for MRSA, E. coli and K. pneumoniae resistant to third-generation cephalosporins, HIV, and tuberculosis. In the most severe scenario modelled, corresponding to a doubling of current infection rates for all the infections included in the study, the authors estimated that by 2050, 700 million deaths would occur as a direct result of resistance, which would inflict a cumulative cost of over $14 trillion to the world economy.
In a similar study, RAND Europe (Taylor et al., Reference Taylor, Hafner and Yerushalmi2014) modelled the effect of resistance on economic production through its negative impact on the labour supply. The model included the same diseases as those considered in the KPMG study, along with malaria. Different scenarios of resistance were compared to a baseline scenario of no resistance in five broad regions across the world. The model estimated that by 2050, relative to a world with no resistance, the total loss of people in productive age would range from 11 million for 5% increase in the current rates of AMR to 444 million under a 100% resistance scenario. This would correspond to a cumulative GDP loss to the global economy of between $2.1 and $58.9 trillion, over a period of 40 years.
Using a general equilibrium model, the World Bank (Adeyi et al., Reference Adeyi, Baris and Jonas2017) assessed the impact of AMR on global GDP and on specific components of the world economy between 2017 and 2050. Two alternative scenarios of low and high AMR impact were simulated as shocks to labour supply. In the optimistic case of low AMR impacts, the simulations estimated that, by 2050, annual global GDP would likely fall by 1.1%, relative to a base-case scenario with no AMR effects. The corresponding GDP shortfall would exceed $1 trillion annually after 2030. In the high AMR impact scenario, the world would lose 3.8% of its annual GDP by 2050, with an annual shortfall of $3.4 trillion by 2030. International trade and livestock production were identified as the two areas, in addition to the health care sector, that may suffer the most due to reduced productivity and reduction in sales. In certain regions, antimicrobials are largely used in the agriculture sector as an alternative to other more expensive options to allow high-density livestock production. Low- and middle-income countries are particularly exposed as livestock production and export represent important sectors of their economies.
Trade may also be affected by lower demand for animal products from consumers and limitations to imports. For example, in 2015, the negative media publicity about infections in poultry caused a 15–20% drop in sales of chickens in Norway which continued for several months (O’Dwyer, Reference O’Dwyer2015). Similar events may also provoke trade disruptions, with countries imposing bans on imports following disease outbreaks. These reactions sharply reduce and disrupt economic activity, particularly in the case of diseases for which no effective cure is available (Brahmbhatt & Dutta, Reference Brahmbhatt and Dutta2008). More broadly, it has been hypothesized that the effect of AMR may follow patterns similar to those of epidemic outbreaks developing into pandemics (Anderson, Reference Anderson1999; Spellberg et al., Reference Spellberg, Guidos and Gilbert2008). If this happened, negative effects and financial losses in the sectors such as tourism and banking could also occur (Jonas, Reference Jonas2013).
Conclusion
AMR is a complex phenomenon that hinders the treatment and prevention of infections and threatens the effective provision of health care. Multiple studies have demonstrated that AMR is associated with increased morbidity and mortality. However, estimating the total health burden of AMR is challenging given the number and types of resistance, microorganisms, sites of infection and hosts. The availability, quality and comparability of data are additional limiting factors.
A common feature of most existing evaluations is the limitation of their scope as they often focus on a specific infectious disease or small set of diseases. In particular, economic evaluation studies have consistently failed to consider “the bigger picture” when it comes to assessing AMR (Smith & Coast, Reference Smith and Coast2013). As discussed earlier in the chapter, this is due to a large extent to the complex nature of the problem of resistance – lack of data, high parameter uncertainty, and unknown long-term consequences of interventions. Studies are easier to perform if their scope is limited to specific resistances or to the “micro” level of individual institutions (Coast et al., Reference Coast, Smith and Karcher2002). This has led to the somewhat paradoxical current situation, where more empirical information on the economic burden of resistance is available, but the value of that information to those in charge of designing and implementing strategies to deal with resistance is limited.
The vast majority of existing economic studies tend to consider the costs and health outcomes due to resistance without comparison. If we define economic evaluation as “the comparative analysis of alternative courses of action in terms of both their costs and consequences” (Drummond et al., Reference Drummond, Sculpher and Claxton2005), most studies on the economic impact of AMR can be considered as partial economic evaluations. They provide valuable and detailed descriptive information in terms of cost and health consequences of resistance. This kind of descriptive work is important but does not provide a complete picture of the problem of AMR either in terms of costs or effects. A more comprehensive understanding of the problem requires an estimate of the “opportunity cost” associated with resistance. That is what society is missing out on by committing resources to dealing with the causes and consequences of resistance and not allocating or using those resources to do something else. To determine that opportunity cost, it is necessary to compare alternative courses of action or events in terms of both their costs and consequences. The slow progress in combating AMR is partly due to an insufficient or poor evidence base for the effectiveness and cost–effectiveness of the many existing policies across the human health and animal sectors (Dar et al., Reference Dar, Hasan and Schlundt2016). In the human health sector, the OECD has recently published a set of analyses evaluating the cost–effectiveness of selected policies to tackle AMR in different countries (OECD, 2018). The results of this work showed that investing and implementing preventive strategies – such as hand hygiene, surface cleaning and stewardship programmes – at the national level, would significantly reduce the health and economic burden of AMR and deliver high value for money. Similar evaluation work is needed to assess the effectiveness and cost–effectiveness of interventions to address AMR in the animal and environmental sectors.