Abstract
Introduction
Accurate formulae to predict the optimal insertion length of endotracheal tubes (ETT) are necessary for safe care and have been based on height, weight, age, and ETT size. We believe height best reflects the somatic growth of the trachea. Our goal is to compare a formula generated using height for optimal initial insertion length of ETT to previously published formulae based on height, weight, age, and ETT size.
Methods
We retrospectively reviewed chest radiographs over a two-year period where the head was assured in midline and midposition. We excluded children with conditions altering tracheal dimensions or stature, and scoliosis. We chose 2 cm above the carina to be the optimal insertion length of the ETT which was then correlated to height. We created linear regression equations and Bland-Altman plots.
Results
Two hundred three orotracheally intubated children were included. The optimal ETT insertion length using the formula Height (cm)/8 + 3.4 had a high association with linear regression and Bland-Altman plots had the narrowest 95% limits of agreement as compared to previously published formulae.
Conclusions
We found optimal insertion length = Height (cm)/8 + 3.4 is more accurate as compared to commonly used formulae that are based on weight, age, or ETT size. This formula places the ETT 2 cm above the carina which should be safe until a chest radiograph is obtained. We find that the use of formulae that divide the height by 10 overestimates the depth of insertion in younger, smaller children and underestimates the depth of insertion in older children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Accurate formulae to predict the optimal insertion length of endotracheal tubes (ETT) are necessary for safe practice in pediatric critical care medicine (PCCM). A mispositioned ETT can result in hypoxia, hypoventilation, atelectasis, pneumothorax, or unplanned extubation [1] and has resulted in tube thoracostomy when breath sounds could not be heard due to endobronchial intubation [2]. Although a chest radiograph will usually be obtained following intubation, this may be delayed for placement of central venous catheters or arterial lines in patients who need further resuscitation. In addition, repositioning an ETT increases the risk of unplanned extubation as the tube is unsecured for a period of time.
Common formulae for optimal insertion length of ETT have been based on height [3], weight [3], age [4], and endotracheal tube diameter [5, 6]. Less common formulae have used foot length [7], middle finger length [8], body surface area [9], and incisor-manubriosternal joint length [10]. Many PCCM practitioners have memorized the simpler formulae. Unfortunately, these formulae may be just sufficient with an incidence of malpositioned endotracheal tubes of 25% or higher when confirmed by radiograph [9, 11, 12]. The ability to use more complex formulae exported from the electronic healthcare record may result in improved accuracy. In our own institution, we have developed a formula for optimal insertion length based on the patient’s height or length measured on admission that is incorporated into the resuscitation sheets placed at every bedside. We used height to create our formula as we understand it best tracks the somatic growth of the trachea [13,14,15]. There is great variability in patient’s weight for age which has been shown to follow a U-shaped distribution in North American PICUs [16]. For example, weight variability is one reason height is used to predict ideal body weight for mechanical ventilation [17]. Age has limitations given syndromic children may be much smaller than anticipated for their stated age [18], but their height tracks the overall growth of the trachea for that child [13, 14]. Formulae for optimal insertion length based on ETT size are a two-step process as the initial ETT size is often based on age. This creates another opportunity for increased error.
We believe the optimal position for the initial placement of an endotracheal tube after intubation is a point 2 cm above the carina. Changes in head position will move the tip of a secured endotracheal tube. However, movement of the ETT tip should not exceed ± 2 cm and therefore are not likely to move an ETT down into a mainstem bronchus position or up and cause an unplanned extubation [19,20,21]. Ideal ETT position may vary by manuscript and institutional preference. However, a position 2 cm above the carina has worked well in our clinical practice with a low unplanned extubation rate.
The goal of this study is to use our new data set to compare a regression formula generated using height for optimal insertion length of ETT to previously published formulae based on height, weight, age, and ETT size.
Methods
All orally intubated children admitted to the PICU at CHLA over a 2-year period were retrospectively screened for inclusion. Radiographs were considered eligible for study if the following criteria were met:
-
(a)
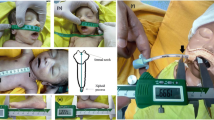
Head midline and mid-position are identified radiographically as the inferior aspect of the soft tissue of the chin intersecting within ± 1 cm of a line drawn from the superior aspects of the acromioclavicular joints bilaterally (Fig. 1A).
-
(b)
Anterior–posterior chest radiographs were obtained per the Radiology Department’s current practice, with the X-ray tube directed perpendicularly toward the mid-thorax.
-
(c)
No evidence of lateral rotation.
A Radiograph demonstrating measurements taken to assure adequacy of radiograph for study. Two lines were drawn, the first intersecting the lateral superior aspects of the clavicles, and the second drawn from the most inferior aspect of the soft tissue shadow of the chin inferiorly, bisecting the first line. The radiograph was considered appropriate if the line intersecting the clavicles was within 1 cm of the bisecting line from the soft tissue shadow of the chin. B Radiograph demonstrating the measurement from the carina to the tip of the endotracheal tube. ETT, endotracheal tube
If the radiograph was appropriate, patient records were reviewed and children with conditions considered likely to influence tracheal dimension (tracheomalacia, vascular ring, mediastinal mass, prior mediastinal radiotherapy), conditions affecting stature (dwarfism), history of abnormal airway anatomy, or scoliosis were excluded. For eligible children, demographic information was extracted. Height/length is measured on admission with the patient in a supine position by the nursing staff in the ICU. Our respiratory therapists (RT) carry out a rigorous ETT securement procedure for all patients as part of a quality assurance bundle developed to prevent unplanned extubation. During the securement, the ETT position is recorded by the RT, and the position is confirmed to be unchanged twice a shift. The centimeter mark on the ETT taped in the middle of the lip is noted as the external distance. For the chest radiographs, we used the digital ruler from our radiograph system to obtain the distance from the carina to the most distal point of the ETT (Fig. 1B). We added this distance to the cm mark on the ETT giving us the upper lip to carina distance. We calculated the optimal insertion length as the distance from the marking on the ETT to the carina minus 2 cm. The optimal insertion length of the ETT was then correlated to height, weight, age, and predicted ETT size based on the modified Cole formulae of [(Age (years) + 16)/4.
Statistical analysis
Normality was assessed with the Shapiro–Wilk test. Linear regression analysis and correlation coefficients (r2) were calculated using patient data. Regression formulae were compared to previously published formulae. Accuracy over the range of optimal insertion length was evaluated with Bland-Altman analysis [22] using patient data. This was performed for our newly generated regression formula for height predicting optimal ETT insertion length as well as for previously published formulae for height, weight, age, and ETT size. The paired differences between the insertion length predicted by these formulae and the optimal insertion length of the ETT were plotted against the mean of the two data values. Bias and standard deviation were calculated, along with the 95% limits of agreement. Values were considered significant if they fell within ± 2 cm from the zero-difference axis which represents no difference between the predicted and optimal lengths. Graphs and analysis were performed using GraphPad Prism version 6 (GraphPad® Software, Boston, MA) and Stata version 15.1 (StataCorp., College Station, TX, USA).
Research involving human participants
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. This study was reviewed and approved by the Institutional Review Board of Children’s Hospital Los Angeles. The need for written informed consent was waived.
Results
A total of 843 orotracheally intubated children were screened over a 2-year period. See Supplemental Figure S1. A total of 203 cases had acceptable and complete data for this analysis. The ages of the children ranged from 2 days to 16.8 years (median 3.4 years; 1.3 IQR 0.8, 9.7); body weights ranged from 2.3 kg to 100.8 kg (median 15.6 kg; 1.3 IQR 8.4, 35.4); heights ranged from 41 to 184 cm (median 98 cm; 1.3 IQR 71, 132); predicted ETT size ([Age (years) + 16]/4) ranged from 4.0 mm ID to 8.0 mm ID (median 5.0 mm ID, 1.3 IQR 4, 6.5).
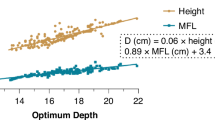
Linear regression formulae related to the radiographically determined optimal insertion length of the ETT were generated (Fig. 2). Results of the new formulae created with correlation coefficients as well as previously reported formulae are shown in Table 1.
Results of the Bland-Altman plots for height formulae generated from patient data as well as previously published formulae are shown in Fig. 3. Optimal ETT insertion length using the formula Height (cm)/8 + 3.4 had the second lowest bias, the lowest SD of the bias, and the narrowest 95% limits of agreement as compared to previously published formulae. These narrow limits of agreement help to confirm what we visually identify that our new formulae for optimal ETT length performed favorably throughout the range of ETT length.
Discussion
Linear regression analysis demonstrated a strong association between patient height and optimal insertion length of the ETT. Linear regression analysis has a lower association for weight and optimal ETT insertion length as compared to height. Linear regression analysis using age or ETT size to insertion length has similar association results to those using height. However, a linear regression analysis is not the only evaluation of the performance of these techniques. A Bland-Altman analysis is used to assess the level of agreement between two methods in the comparison of a new technique to an established one. The Bland-Altman analysis serves as a valuable statistical tool because it assumes that neither system is a “gold standard”; it is simply a comparison between the two systems. By Bland-Altman analysis, there is a consistent trend for the previously published equations to overestimate the optimal insertion length of the ETT at lower values and underestimate the optimal insertion length at higher values. The Bland-Altman plots using the various regression formulae from our data predicting optimal length from the child's height, Optimal Insertion Length = Height (cm)/8 + 3.4, has the narrowest 95% limits of agreement in comparison to the other formulae. It is also the only predictive equation that does not demonstrate significant length-dependent variation.
Other investigators have also shown inaccuracies when using predictive equations from weight, age, or ETT diameter to create formulae for optimal ETT length of insertion [23,24,25]. There has been a progression of papers published using updated clinical data to evaluate the accuracy of the formulae from Cole (age/2 + 12) and Morgan (height cm/10 + 5) [3, 4]. It should be noted that Cole’s formulae for both ETT size and insertion depth were designed to assist the pediatric anesthesiologist, and they had no associated validation or derivation from a data set that can be found. Further, we might respectfully now, in more modern times, disagree with Dr. Cole’s words in his manuscript “Formulas more nearly perfect than these would lose infinitely more through awkwardness than the little that might be gained in accuracy” Like the Cole formulae, the Morgan formula has remarkably stood the test of time. However, in our dataset as opposed to the Morgan formula we find it best to divide height by 8. In reviewing further studies, we realize this has previously been demonstrated by Hunyady et al. [26]. Their study was designed to create a formula for the distance from the front teeth-to-carina (FT-C). Their 170 patients were undergoing anesthesia for cardiac catheterization where fluoroscopy was being used. Their reported FT-C formula was 0.12 * height (cm) + 5.2. This is equivalent to height (cm)/8.3 + 5.2 to the carina. The goal of our study was to put the ETT in a safe tracheal position for ICU patients. Our standard practice is for this to be a point 2 cm above the carina. If 2 cm is subtracted from the formula by Hunyady et al. (to the carina) the equation becomes nearly the same formula as ours (to a point 2 cm above). From the review of our Bland-Altman analysis as well as the study by Hunyady et al., we argue that predictive formulae where height is divided by 10 are simpler but less accurate.
Routine chest radiographs are obtained in an ICU setting as malposition is a frequent finding [12, 27,28,29,30]. ETT malposition likely contributes to unplanned extubation which can be a significant source of morbidity [31,32,33,34]. There is no established standard describing the head position as well as the optimal position of the tip of the ETT as determined by radiography. We assigned the distance from the upper lip to 2 cm above the carina as the optimal ETT insertion length, as this position has been supported by prior studies [35, 36]. We note that there is movement of ETTs cephalad and caudad with extension and flexion of the neck, respectively [19,20,21, 37]. Sugiyama et al. [19] demonstrated the movement of 0.9 cm on flexion and 1.7 cm on the extension in their group of toddlers, further supporting the choice of 2 cm above the carina as being an appropriate distance. We recognize that other institutions, based on experience and training, may have a different position where they place the tip of the ETT. As there is no gold standard, we can only identify our reasoning behind 2 cm above the carina.
The initial formulae we used in the ICU were created by pediatric anesthesiologists. The stability of children in the operating room allows mainstem intubation and then subsequent withdrawal to determine optimal position [35, 36]. A study by Mariano, et al. compared three methods for estimating appropriate tracheal tube depth in children, including deliberate mainstem intubation with the subsequent withdrawal of 2 cm, alignment of the double black line marker of the tube at the vocal cords, and placement using the modified Yates formulae of 3 × (predicted ETT size for age) [38]. They demonstrated that deliberate mainstem intubation yielded the most reliable results [38]. Further studies reported such techniques as bronchoscopy [3, 19], Trachlite [39], and ultrasonography [35, 40],for assessing optimal tube placement. These methods are more difficult when the patient is being intubated for respiratory failure. These methods require additional equipment or expertise. Further, one-lung ventilation, even if temporary, may provoke hemodynamic instability in an ICU setting. Finally, we have concerns about choosing the optimal insertion length of endotracheal tubes based on the intubation depth markings on the endotracheal tube [41]. The position of these markers vary by brand [42, 43] and this method requires an optimally sized endotracheal tube which may have limitations based on choosing size by age.
As this was a retrospective study, personnel were not present when the radiographs were obtained. Though it is standard practice for the radiology technician to center the beam appropriately, there is considerable room for variability in technique. This study attempts to minimize this error by selecting patients and radiographs based on strict criteria as outlined earlier. However, there is room for error if the focus of the beam shifts cephalad or caudad, as this will affect the projection of the endotracheal tube onto the film, causing the tip of the tube to appear either lower or higher with respect to the carina. Following intubation, we cannot assure that the ETT was not curled in the back of the mouth at the time that the chest radiograph was taken. We cannot assure the accuracy of the height measured in a supine position as compared to standing. Given the acuity of patients in an ICU setting, it may not be possible to have the most accurate height, but this can remain true for weight or other variables. Finally, we recognize that positioning the ETT 2 cm above the carina may not reflect the practice of other ICUs. The choice by each individual for the best position will reflect their training and experience. This position has worked well for us clinically but there is no gold standard. Finally, in the relatively small sample size physiologic data such as height was not normally distributed, and therefore, not all of the assumptions of regression analysis could be met.
Conclusion
The analysis of our data demonstrated that the use of the formula: Optimal insertion length = Height (cm)/8 + 3.4 is more accurate as compared to commonly used formulae that are based on weight, age, or ETT size. Our choice to position the tip of the ETT 2 cm above the carina reflects our ICU practice to prepare for the movement of the head that will reposition the ETT tip cephalad or caudad. Our findings are similar to a formula created by Hunyady et al. where the distance from the front teeth to carina = Height (cm)/8.3 + 5.2. We find that the use of formulae that divide the height by 10 overestimates the depth of insertion in younger, smaller children and underestimates the depth of insertion in older children. We argue that formulae for ETT position should be based on accuracy and not simplicity and as every cell phone has a calculator app we no longer need to rely on mental calculations for quick results.
Availability of data and materials
Data is available on request due to privacy.
References
Miller KA, Kimia A, Monuteaux MC, Nagler J (2016) Factors associated with misplaced endotracheal tubes during intubation in pediatric patients. J Emerg Med 51:9–18. https://doi.org/10.1016/j.jemermed.2016.04.007
Rost F, Donaubauer B, Kirsten H et al (2022) Tracheal tube misplacement after emergency intubation in pediatric trauma patients: a retrospective, exploratory study. Children 9:289. https://doi.org/10.3390/children9020289
Morgan GA, Steward DJ (1982) Linear airway dimensions in children: including those from cleft palate. Can Anaesth Soc J 29:1–8. https://doi.org/10.1007/BF03007939
Cole F (1957) Pediatric formulas for the anesthesiologist. Am J Dis Child 94:672–673. https://doi.org/10.1001/archpedi.1957.04030070084009
(2000) Part 10: Pediatric advanced life support. Circulation 102. https://doi.org/10.1161/circ.102.suppl_1.I-291
Yates AP, Harries AJ, Hatch DJ (1987) Estimation of nasotracheal tube length in infants and children. Br J Anaesth 59:524–526. https://doi.org/10.1093/bja/59.4.524
Embleton ND, Deshpande SA, Scott D et al (2001) Foot length, an accurate predictor of nasotracheal tube length in neonates. Arch Dis Child Fetal Neonatal Ed 85:F60-64. https://doi.org/10.1136/fn.85.1.f60
Zhuang P-E, Lu J-H, Wang W-K, Cheng M-H (2023) A new formula based on height for determining endotracheal intubation depth in pediatrics: a prospective study. J Clin Anesth 86:111079. https://doi.org/10.1016/j.jclinane.2023.111079
Neunhoeffer F, Wahl T, Hofbeck M et al (2016) A new method for determining the insertion depth of tracheal tubes in children: a pilot study. Br J Anaesth 116:393–397. https://doi.org/10.1093/bja/aev545
Jain A, Wadhwa B, Saxena KN (2020) Preventing inadvertent endobronchial intubation: upper incisor to manubriosternal joint length as a predictor of airway length in children. Paediatr Anaesth 30:1240–1244. https://doi.org/10.1111/pan.14023
Phipps LM, Thomas NJ, Gilmore RK et al (2005) Prospective assessment of guidelines for determining appropriate depth of endotracheal tube placement in children*. Pediatr Crit Care Med 6:519–522. https://doi.org/10.1097/01.PCC.0000165802.32383.9E
Volsko TA, McNinch NL, Prough DS, Bigham MT (2018) Adherence to endotracheal tube depth guidelines and incidence of malposition in infants and children. Respir Care 63:1111–1117. https://doi.org/10.4187/respcare.06024
Yamamoto T, Schindler E (2022) Ideal depth of endotracheal intubation at the vocal cord level in pediatric patients considering racial differences in tracheal length. J Clin Med 11:864. https://doi.org/10.3390/jcm11030864
Butz RO (1968) Length and cross-section growth patterns in the human trachea. Pediatrics 42:336–341
Thorne Griscom N, Wohl MEB, Fenton T (1989) Dimensions of the trachea to age 6 years related to height. Pediatr Pulmonol 6:186–190. https://doi.org/10.1002/ppul.1950060312
Ross PA, Newth CJL, Leung D et al (2016) Obesity and mortality risk in critically ill children. Pediatrics 137:e20152035. https://doi.org/10.1542/peds.2015-2035
Brower RG, Shanholtz CB, Fessler HE et al (1999) Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 27:1492–1498. https://doi.org/10.1097/00003246-199908000-00015
Zemel BS, Pipan M, Stallings VA et al (2015) Growth charts for children with Down syndrome in the United States. Pediatrics 136:e1204–e1211. https://doi.org/10.1542/peds.2015-1652
Sugiyama K, Yokoyama K (1996) Displacement of the endotracheal tube caused by change of head position in pediatric anesthesia: evaluation by fiberoptic bronchoscopy. Anesth Analg 82:251–253. https://doi.org/10.1097/00000539-199602000-00006
Jin-Hee K, Ro Y-J, Seong-Won M et al (2005) Elongation of the trachea during neck extension in children: implications of the safety of endotracheal tubes. Anesth Analg 101:974–977. https://doi.org/10.1213/01.ane.0000169330.92707.1e
Kim J-T, Kim H-J, Ahn W et al (2009) Head rotation, flexion, and extension alter endotracheal tube position in adults and children. Can J Anesth/J Can Anesth 56:751–756. https://doi.org/10.1007/s12630-009-9158-y
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160. https://doi.org/10.1191/096228099673819272
Ebenebe CU, Schriever K, Apostolidou S et al (2023) Recommendations for endotracheal tube insertion depths in children. Emerg Med J 40:583–587. https://doi.org/10.1136/emermed-2022-212494
Santos DLS, Andrade PDDO, Gomes ELDFD (2020) Does the endotracheal tube insertion depth predicted by formulas in children have a good concordance with the ideal position observed by X-ray? Rev Bras Ter Intensiva 32:295–300. https://doi.org/10.5935/0103-507X.20200046
Khanna P, Garg H, Ray BR et al (2021) Accuracy of predictive equations in guiding tracheal intubation depth in children: a prospective study. Pediatr Anesth 31:1304–1309. https://doi.org/10.1111/pan.14301
Hunyady AI, Pieters B, Johnston TA, Jonmarker C (2008) Front teeth–to–carina distance in children undergoing cardiac catheterization. Anesthesiology 108:1004–1008. https://doi.org/10.1097/ALN.0b013e3181730288
Kuhns LR, Poznanski AK (1971) Endotracheal tube position in the infant. J Pediatr 78:991–996. https://doi.org/10.1016/S0022-3476(71)80429-8
Quasney MW, Goodman DM, Billow M et al (2001) Routine chest radiographs in pediatric intensive care units. Pediatrics 107:241–248. https://doi.org/10.1542/peds.107.2.241
Harris EA, Arheart KL, Penning DH (2008) Endotracheal tube malposition within the pediatric population: a common event despite clinical evidence of correct placement. Can J Anesth 55:685–690. https://doi.org/10.1007/BF03017744
Im DD, Ross PA, Hotz J, Newth CJL (2019) Evaluating the practice of repositioning endotracheal tubes in neonates and children based on radiographic location*. Pediatr Crit Care Med 20:1057–1060. https://doi.org/10.1097/PCC.0000000000002053
Al-Abdwani R, Williams CB, Dunn C et al (2018) Incidence, outcomes and outcome prediction of unplanned extubation in critically ill children: An 11 year experience. J Crit Care 44:368–375. https://doi.org/10.1016/j.jcrc.2017.12.017
Wollny K, McNeil D, Moss SJ et al (2023) Unplanned extubations requiring reintubation in pediatric critical care: an epidemiological study. Pediatr Crit Care Med 24:311–321. https://doi.org/10.1097/PCC.0000000000003167
Fitzgerald RK, Davis AT, Hanson SJ (2015) Multicenter analysis of the factors associated with unplanned extubation in the PICU. Pediatr Crit Care Med 16:e217–e223. https://doi.org/10.1097/PCC.0000000000000496
Lucas Da Silva PS, Fonseca MCM (2017) Incidence and risk factors for cardiovascular collapse after unplanned extubations in the pediatric ICU. Respir Care 62:896–903. https://doi.org/10.4187/respcare.05346
Bloch EC, Ossey K, Ginsberg B (1988) Tracheal intubation in children: a new method for assuring correct depth of tube placement. Anesth Analg 67:590–592
Kim KO, Um WS, Kim CS (2003) Comparative evaluation of methods for ensuring the correct position of the tracheal tube in children undergoing open heart surgery*. Anaesthesia 58:889–893. https://doi.org/10.1046/j.1365-2044.2003.03336.x
Weiss M, Knirsch W, Kretschmar O et al (2006) Tracheal tube-tip displacement in children during head-neck movement—a radiological assessment. Br J Anaesth 96:486–491. https://doi.org/10.1093/bja/ael014
Mariano ER, Ramamoorthy C, Chu LF et al (2005) A comparison of three methods for estimating appropriate tracheal tube depth in children. Pediatr Anesth 15:846–851. https://doi.org/10.1111/j.1460-9592.2005.01577.x
Hayakawa Y, Iizawa A, Iida H, Dohi S (2001) Finding appropriate endotracheal tube position by Trachlight in children. Masui 50:175–178
Jaeel P, Sheth M, Nguyen J (2017) Ultrasonography for endotracheal tube position in infants and children. Eur J Pediatr 176:293–300. https://doi.org/10.1007/s00431-017-2848-5
Weiss M, Balmer C, Dullenkopf A et al (2005) Intubation depth markings allow an improved positioning of endotracheal tubes in children. Can J Anesth 52:721–726. https://doi.org/10.1007/BF03016560
Goel S, Lim S (2003) The intubation depth marker: the confusion of the black line. Pediatr Anesth 13:579–583. https://doi.org/10.1046/j.1460-9592.2003.01103.x
Singh N, Mohanty CR, Rao PB (2019) Ambiguous pediatric endotracheal tube intubation depth markings: a need for standardization. Korean J Anesthesiol 72:614–615. https://doi.org/10.4097/kja.d.19.00006
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
Conceptualization, data curation, formal analysis, investigation, writing, review, and editing: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted with the approval of the Institutional Review Board of Children’s Hospital Los Angeles. A waiver of written informed consent was approved. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Figure 1.
Flow diagram of patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ross, P.A., Abou-Zamzam, A. & Newth, C.J.L. Height best predicts the optimal insertion length of orotracheal tubes in children. Intensive Care Med. Paediatr. Neonatal 2, 6 (2024). https://doi.org/10.1007/s44253-024-00032-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-024-00032-7