Abstract
Cyclin-dependent kinase 4/6 (CDK4/6) acts as a crucial point of regulation in the G1-to-S transition in the cell division cycle, its aberrant activation was found in various human cancers, leading to abnormal cell proliferation. Recent clinical trials have reported that combined with other small-molecule targeted therapies, CDK4/6 inhibitors increase overall survival and objective response rates in breast cancer (BC), non-small cell lung cancer (NSCLC), and head and neck squamous cell carcinoma (HNSCC). Notably, targeting CDK4/6 triggers an antitumor immune response, providing a potential combined application method for immunotherapy. In this review, we summarize underlying mechanism of targeting CDK4/6 in regulating antigen presentation, immune cell activation, and tumor immune microenvironment (TIME) remodeling and in producing synergistic effects with immune checkpoint blockade (ICB) in cancer clinical treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
Aberrant cell proliferation is a notable cancer hallmark which is characterized by dysregulated mitotic cell cycle progression [1]. The canonical mitotic cell cycle comprises four highly conserved phases: the mitosis (M), DNA synthesis (S), first gap (G1) and second gap (G2) phases. A variety of cyclins and cyclin-dependent kinases (CDKs), such as CDK4, CDK6 and CDK2, regulate the cell division cycle progression in the G1 and G2 phases, determining whether entering the S phase (G1-restriction point) and performing mitosis [2, 3].
CDK4/6 forms a complex with of Cyclin D family members (D1, D2 and D3). Multiple transcriptional regulatory pathways for Cyclin D are closely linked to kinases activity, which is crucial for cell cycle [4]. Specifically, the Cyclin D1–CDK4/6 complex phosphorylates retinoblastoma (Rb) and releases the transcription factor E2F, stimulating related genes transcription needed for cell cycle and for DNA replication [5]. Dysfunction in the Cyclin D1–CDK4/6–Rb axis is constantly observed in various cancers [6,7,8]. Surprisingly, researchers found that besides regulating the cell cycle and impeding tumor growth, CDK4/6 inhibitors (CDK4/6i) also augment immunogenicity of tumor cells, enhance the antigen-presenting cells (APCs) function, re-accommodate immune effector cells and immune-suppressing cells, and equilibrate senescence-associated secretory phenotype (SASP) and cytokine secretion, thus promote the aging of tumor cells and alter the tumor immune microenvironment (TIME), suggesting that antitumor immune response is vital to enhancing CDK4/6-targeted therapy efficacy.
Agents used for immune checkpoint blockade (ICB), particularly the programmed cell death protein-1 (PD-1) and programmed death - ligand1 (PD-L1) monoclonal antibodies, are approved for cancer therapy with sustainable therapeutic benefits [9]. ICB aims to eliminate tumor cells by restoring recognition and killing function of regular T cell, thus avoiding the occurrence of immune escape [10, 11]. Numerous studies have reported that CDK4/6-targeted therapy combined with ICB has an immunological effect that occurs via PD-L1 expression regulation or the elimination of innate and acquired resistance [12].
Thus, inhibition of CDK4/6 may prevent immune evasion through multiple pathways and promote broad-spectrum antitumor immunotherapy. In this review, we briefly summary the role of aberrant CDK4/6 and related signaling during tumor progression, focusing on the critical immunomodulatory roles of targeting CDK4/6 in anticancer therapy. In addition, the potential synergistic mechanism of CDK4/6i and ICB is summarized to help clinicians become more confident in their application of CDK4/6i in antitumor immunotherapy.
2 Targeting CDK4/6 in antitumor therapy
Cyclin D1 transcription is intricately related to multiple signaling pathways, and CDK4/6 serves as a sentinel that integrates diverse signaling cascades in the initiation and progression of the cell cycle [13, 14]. In noncancer cells, extracellular signals can stimulate or inhibit the complex activity of Cyclin D–CDK4/6. However, the aberrant complex activation is independent of mitotic stimulation in cancer cells, leading to uncontrolled proliferation [5].
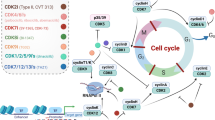
CDK4/6i suppress the Rb phosphorylation, leading to cell cycle arrest [15]. Moreover, as reported by early studies, CDK4/6 coexists with classical tumor signaling pathways such as the Wnt, Myc and p53 pathways [16,17,18]. Ma et al. mentioned that high expression of Myc degrades Rb phosphorylation to improve CDK4/6i therapeutic efficacy [19, 20]. Zhu and his colleagues found that in PARP inhibitors-resistant triple-negative breast cancer (TNBC) cell lines, activated Wnt signaling pathway and overexpressed hyperphosphorylation of β-catenin at the Ser675 site could be strongly inhibited by combining olaparib with palbociclib [21]. Huang et al. confirmed that CDK4/6i induced DNA damage, leading to failure of cells to complete DNA replication in a p53-dependent manner [22]. Researchers further found that prolonged stimulation creates replication pressure in a panel of p53-null tumor cells, contributing to the process of cycle extension instead of complete G1 arrest [23].
Additionally, the function of the CDK4-cyclin D complex is modulated partly via the RTK signaling axis [24, 25]. For instance, the PI3K–AKT–mTOR and RAS–MEK–ERK pathways were found to be excessively activated in palbociclib-resistant BC cells [26, 27]. Kohlmeyer et al. furtherly mentioned that dual inhibition of CDK4/6-MEK emerged novel antitumor activity in Malignant peripheral nerve sheath tumors (MPNST) by inducing plasma cell-associated immune response [28]. In patient-derived organoid and xenograft ovarian cancer (OC) models, CDK4/6i combined with specific PI3K inhibitors (BYL3) and mTOR inhibitors (everolimus) showed effective synergy [29, 30], thereby restoring sensitivity to single-agent exposure, inducing stronger senescence and apoptosis and more effectively inhibiting tumor proliferation [31].
It is therefore more theoretically sound to combine CDK4/6i with other small-molecule inhibitors when treating cancers (Fig. 1). The functions of current CDK4/6i are summarized in detail (Table 1).
3 Immunomodulatory function of targeting CDK4/6 in cancer
The TIME is a complex biosystem, comprises mainly tumor cells, surrounding immune and inflammatory cells, tumor-associated fibroblasts, and various cytokines and chemokines [32, 33]. Many clinical studies have confirmed that inhibition of CDK4/6 has an immunomodulatory effect via its prevention of tumor immune evasion and regulation of the TIME, affecting the combined efficiency. Here, we discuss mainly the different immune response processes induced by targeting CDK4/6 therapy in the TIME.
3.1 Effects on the process of antigen presentation
In addition to inducing cell cycle arrest, CDK4/6i also enhance T cell-mediated antigen presentation by modulating major histocompatibility complex (MHC) I and II expression, APCs function, and costimulatory molecules secretion, thereby inhibiting immune evasion [34].
3.1.1 MHC-I/II
On the tumor cell surface, the lack of MHC expression and molecular presentation function are the most critical factors that promote tumor cell immune evasion [35, 36]. MHC class I and II present polypeptides respectively recognized by CD8+ T-cells and CD4+ T-cells, binding with T cell receptors and activating killing functions [37, 38]. Multiple relative and absolute quantitative immunopeptidomics analyses also revealed alterations in the MHC-I repertoire caused by inhibition of CDK4/6 [39]. This finding has been confirmed in several tumor cell lines, such as BC, melanoma, primary effusion lymphoma (PEL) and Epstein-Barr virus-positive Burkitt lymphoma (BL). Abemaciclib, palbociclib, and ribociclib can upregulate MHC-I/II expression on the surface, making tumor cells easier to identify by an immunological surveillance system [40, 41].
Moreover, Lu and colleagues proved that Rb acts in rescuing interferon (IFN)-γ-induced MHC-II gene transcription in BC cells [42]. Zhu et al. furtherly demonstrated that in mouse embryonic fibroblasts (MEFs), the induction of IFN-γ at the MHC-II Aβ locus is dependent on Rb, inducing antigen presentation to CD4+ T-cells, while other genes, such as Aα and Eβ, are independent of Rb [43, 44]. Liu and colleagues studied the mechanisms underlying palbociclib-induced immune responses in OC. Palbociclib caused an increase in antigen presentation-related gene expression levels, which was partially reversed by small interfering STING RNA [45, 46], and decreased the level of endogenous DNA damage [47]. Thus, palbociclib induces an immune response partially dependent on STING signaling.
3.1.2 APCs and costimulatory molecules
APCs including dendritic cells (DCs) and B cells, macrophages, among others, are carriers that coactivate T-cell recognition ability due to costimulatory molecules [48]. Charles et al. detected positive T-cell responses by ELISpot after T-cell stimulation with peptide-pulsed APCs, founded CDK4/6i-induced human leukocyte antigen (HLA) ligands and maturation of monocytic DCs had a higher capacities for T-cell recognition [49, 50]. Adoptively transferring differentiated DCs in vitro achieved restoration of the DCs compartment in mice treated with CDK4/6i and ICB therapy, resulting in potent tumor inhibition [51]. The study also revealed an inclination towards an increased level of colony-stimulating factor 2 (CSF2) during abemaciclib treatment, which is crucial in promoting M1 polarization and MHC-II expression, as well as the mature of DCs [52].
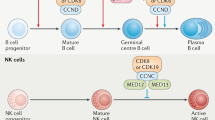
Consistently, Wu and colleagues further reported that incubation of T cells with CDK4/6i-treated PEL and BL cell lines significantly upregulated the surface costimulatory molecules ICAM-1 and B7-2 (CD86, ligand of cytotoxic T lymphocyte-associated antigen-4, CTLA-4), thus enhancing T-cell activation and recognition [41]. Meanwhile, in melanoma cells, CDK4/6i alone or together with MEK inhibitors increased the T-cell costimulatory molecules CD103 and CD137 expression [53]. Furthermore, a comprehensive understanding from two independent phase I clinical trials (NCT02778685, NCI02648477) analyzed subjects’ peripheral blood mononuclear cells by high-parameter flow cytometry to assess baseline immune subset composition and longitudinal changes after therapy and found that palbociclib-treated patients had increased levels of CD83 and human leukocyte antigen-DR (HLA-DR), indicating enhanced antitumor maturity and cDC antigen-presenting capacity [54] (Fig. 2).
Functions of targeting CDK4/6 in antigen presentation. Inhibition of CDK4/6 up-regulates cell-surface MHC moleculesby stimulating IFN-γ secretion or activating the ubiquitination pathway and the STING pathway, as well as facilitates the antigen presentation to CD8 + T cells and CD4 + T cells under the action of various stimulating factors
Therefore, CDK4/6i increase the immunogenicity of tumor cells surface and improve the presentation function of APCs by simulating costimulatory molecules, providing new ideas for activating CDK4/6i in immunotherapy.
3.2 Function and distribution of immune cells
In addition to promoting antigen presentation in T-cells, inhibition of CDK4/6 can indirectly or directly affect immune cells and promote corresponding immune responses. TCGA analysis indicated a negative relevance between immune cell abundance and CDK4/6 expression in cytotoxic cells, Th1 cells, CD8+ T cells, NK cells, and CD56 bright cells [45]. Here, we discuss mainly the immune cell modulatory mechanisms of targeting CDK4/6 therapy from immune effector and suppressing cells.
3.2.1 Immune effector cells
Several studies have shown that CDK4/6i induce CD8+ T cell, CD4+ T cell, and B-cell activation and recruitment in TIME [55, 56]. A clinical retrospective study (NCT02499770) showed that brief exposure to trilaciclib during chemotherapy not only preserves and increases peripheral blood lymphocyte counts but also significantly predicts prognosis and survival by increasing CD8+ T cells and CD4+ T cells in SCLC patients [57, 58]. Chimeric Antigen Receptor T-Cell Therapy (CAR T-cell therapy) plays a novel adoptive antitumor immunomodulatory role by arming T cells or increasing the immunogenicity of tumor cells. Thus CDK4/6i could be a promising adjuvant for CAR T-cell therapy because of its immunomodulatory effects.
Deng and colleagues isolated CD4+CD25− T cells from tumor-bearing and naïve mice under CD3 and CD28 stimulation and assessed IL-2 production from T-cells to further explore effector T-cell regulatory mechanisms. Finally, inhibition of CDK6 results in high activation of NFAT4 signaling in relation to IL-2 secretion and upregulation of activation markers of T-cells, such as 4-1BB (Tnfrsf9), Icosl, GITR (Tnfrsf18), CD40, and CD86 [59]. Specifically, NFAT4 is needed to activate T-cells and transcriptionally regulating IL-2, while CDK6 can phosphorylate and inactivate NFAT4 [60].
In addition, authors also observed an increased persistence and memory effect in parallel with CD8+ tumor-infiltrating lymphocyte (TIL) enrichment, which occurs regardless of whether APCs activate T-cells [56]. Higher expression of TCF1, CD127, and CD147, as memory markers, and CD8+ TILs was the most effective effector cell [61, 62]. Veiga-Fernandes and colleagues further demonstrated the mechanism of memory cell formation. The G0/G1 status of memory T-cells is different from that of naïve cells, as it is characterized by lower p27Kip1 expression and higher CDK6 kinase activity [63]. Heckler et al. also considered that it not only relies on arrest of cell cycle but is also related to MXD4-mediated Myc inhibition after CDK4/6i treatment [64]. Notably, although CDK4/6i can promote effector T-cell infiltration and activation, they suppress cell proliferation, indicating that these targeted agents may have complementary effects when used with antitumor immunotherapy.
3.2.2 Immune-suppressing cells
Immune suppressing cells consist of regulatory T-cell (Treg), M2 macrophages, regulatory B cell (Breg), among others. A series of studies demonstrated that treating metastatic BC patients with CDK4/6i led to a global reduction in Tregs (CD4+CD25+FOXP+), polymorphonuclear bone and monocyte marrow-derived suppressor cells (CD66b+HLA−DR−), thereby attenuating the immunosuppressive TIME [65]. Additionally, after 12 weeks of treatment with ribociclib (NCT03096847), immune-related signature gene expression analysis and deep T-cell receptor profiling showed that Helios+ Tregs declined more significantly than did Helios− Tregs [66]. This demonstrated that an activated phenotype of Tregs was more easily induced by CDK 4/6 inhibitors, which more effectively inhibit the proliferation of autoreactive splenocytes.
To explore how CDK4/6 is involved in the downregulation of Tregs, Xu and colleagues reported that when mice were treated IL-33, CDK4 and Cyclin D1 expression significantly increased, and hepatic Tregs proliferated, indicating that IL-33-induced Treg cell proliferation was caused by overexpression of CDK4 and Cyclin D1 [67]. Additionally, according to the TCGA data, CCND1 is also linked to an increased ratio of immune suppressor cells. CCND1-amplified melanoma murine models were also found to have notably reduced expression of CD8, Gzm, B2M, and Tap1, which resulted in decreases in the number of plasma cell, CD8+ T cell, T follicular helper cell and an increase in Treg [68].
Overall, after targeted CDK4/6 therapy, the proportion of Treg/CD8+ T cells significantly declined, indicating a shift toward antitumor immunity in the immune balance. The short-term exposure to CDK4/6i facilitated long-term effect of endogenous antitumor T-cell immunity, thereby augmenting the persistence and therapeutic efficacy of CAR T-cell therapy.
3.3 Modulation of cytokines in the TIME
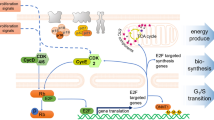
Cytokines are key elements that coordinate TME and control tumor-immune cell interactions. A series of studies demonstrated that CDK4/6i can modulate the TIME by influencing cytokine expression and secretion, thereby augmenting the responsiveness of antitumor immunotherapy [69]. For instance, in OC patient peripheral blood treated with abemaciclib, immune effector cytokines, including IL9, IL3, CXCL16, CRG-2, TNFSF8, and CXCL13, etc. were up-regulated, while three immune suppressive factors (TCK-1, MMP3, SDF1-α) were downregulated [55, 70]. (Fig. 3).
The role of CDK4/6 inhibitors in remodeling TIME. Targeting CDK4/6 promotes immune effector cells through the Mxd4-Myc pathway, stimulates IL-2 secretion by NFAT4, and inhibits immune suppressive cells through the Rb-dependent pathway and the IL-33-MyD88 axis, promoting the secretion of some stimulating factors or inhibiting some factors release to remodel the TIME
3.3.1 Aging-related secretory phenotypes
A typical biological endpoint of CDK4/6 inhibition therapy is induced aging. Senescent cells undergo stable cell cycle arrest and fail to proliferate but can initiate complex proinflammatory responses and the enhanced expression of several secretory cytokines, known as SASP [71]. In A673, SK-ES-1, SK-N-MC, and Ewen's sarcoma (ES) cell lines, abemaciclib can upregulate the expression of CCL2/MCP-1 and IL8, which are biomarkers of therapy-induced senescence (TIS) [72], and stimulate the interferon pathway to promote the inflammatory immune response and remodel the inflammatory microenvironment [73, 74]. Interestingly, Lee et al. found that CDK4/6i consistently induce more SASP secretion and maintain higher expression than DNA-damaging agents-induced cells [75]. Anna and colleagues also showed that in melanoma PDX models, inhibition of CDK4/6 confers CCL5 secretion in an NF-κB-dependent manner and facilitates the recruitment of tumor-infiltrating leukocytes (TILs), finally leading to senescence [71, 76].
3.3.2 Chemokines
Cytokines and their receptors have been extensively studied as poteintial anti-tumor therapeutic strategies. CDK4/6 inhibition therapy can enhance the activity and chemotaxis of immune effector cells by inducing chemokine secretion. In an experiment by Uzhachenko et al., CDK4/6i were beneficial to T cell therapies like adoptive T cell transfers and T cell activating antibodies by activating chemokines CCL5, CXCL9, and CXCL10, thus recruiting activated CD8+ T-cells in BC mouse models [77]. According to Peuker et al., ribociclib downregulates immunosuppressive chemokines (CCL2, CCL7, CCL22) and then drives immunosuppressive cell chemotaxis and differentiation, including Tregs, MDSCs, and macrophages [66, 78]. Moreover, secretion of these immunosuppressive chemokines also indicates a poor prognosis in BC patients [79].
Taken together, the TIME is induced to a favorable immune-prohibitory tumor microenvironment with a more immune initiation state and improved patient survival. CDK4/6i may be a reliable clinical tool either as adjuvants to reinforce antitumor function or as immunomodulatory agents such as targeting cytokine and its receptor therapy.
4 CDK4/6 inhibition augments ICB sensitivity
Currently, anti-PD-1/PD-L1 monoclonal antibody (mAb) has made an achievement worthy of celebration in tumor immunotherapy. Despite the improvements in progression-free survival, the response efficacy in some patients remains unsatisfactory [80, 81]. Emerging evidence suggests that CDK4/6i could augment anti-PD-L1 agent sensitivity and overcome either innate or acquired resistance to ICB therapy. Combining CDK4/6i with ICB has shown decent therapeutic effects in existing clinical trials, which is summarized as follows (Table 2).
4.1 Synergistic effect of CDK4/6 inhibitors and ICB
In combination with anti-PD-1 therapy, abemaciclib has synergistic effects according to meaningful factor analysis and survival analysis [55, 82]. Abemaciclib monotherapy in a CT26 BALB/c mouse model has demonstrated the capacity to impede tumor growth by augmenting the inflammatory signature of T-cells within the TIME. Combined with anti-PD-L1 mAb, it can generate complete regression of tumors, generate immune memory, and enhance cell cycle control [74]. Subsequently, Jang et al. also claimed that combination therapy results in a more pronounced tumor inhibition and superior survival time compared to anti-PD-1 mAb monotherapy [83].
A series of studies related to the regulatory mechanism noted that the CyclinD1-CDK4/6-Rb axis participates in the direct or indirect regulation of PD-L1 expression and thus has an auxiliary effect during immunotherapy. Jin et al. knocked down Rb with two independent shRNAs in PC-3 cells and found that phosphorylated Rb can directly bind to NF-κB and its promoter and then promote PD-L1 transcription [80]. Moreover, in the prostate cancer cell line LNCaP, NF-κB and Rb phospho-mimetic peptides activated the expression of the inflammatory factor TNF-α, thereby indirectly upregulating PD-L1 mRNA levels [84, 85]. A combination treatment regimen of avelumab, palbociclib, and cetuximab is well tolerated in patients with R/M HNSCC in a phase I study and warrants further study [90]. All this evidence suggests that combining CDK4/6i and ICB may confer a greater benefit than either agent alone.
4.2 CDK4/6 inhibitors eliminate both innate and acquired resistance to ICB
4.2.1 Innate resistance
Regulator of chromosome condensation 1 (RCC1) has been implicated by Zeng et al. in the mechanism of inherent ICB resistance in lung adenocarcinoma. In their in vitro trials, knocking down RCC1 hindered the proliferation of H1299 and A549 cells and downregulated CDK4 and p-Rb protein expression levels, thus upregulating PD-L1 expression and reducing resistance to ICB therapy [86]. However, whether using CDK4/6i in PD-L1-resistant tumors with high RCC expression can block RCC's regulation of PD-L1, resulting in excellent combination efficacy, remains to be investigated. RNA-Seq analyses have also suggested that CDK4 abnormalities may also contribute to aberrant PD-L1 expression, as evidenced by PD-L1-positive rates for CDK4-gain patients being lower than those for CDK4-normal patients. The authors further explored AMC-3 (CDK4 gain) and SK-MEL-5 (CCND1 gain) melanoma cell lines and found that treatment with palbociclib increased PD-L1 protein levels [87].
4.2.2 Acquired resistance
Wang et al. reported that in NSCLC, acquired drug resistance to anti-PD-1 mAb may be attributed to the acquisition of a homozygous deletion of B2-microglobulin (B2M), an essential protein for MHC-I antigen presentation, resulting in loss of surface MHC-I expression [88, 89]. Ribas et al. also demonstrated that this kind of resistance is due to the lack of memory T-cells and re-exhausted Tex cells [90, 91]. However, tumors with CCND1 amplification exhibited either a reduction in B2M expression or a remarkable increase in quiescent CD4+ memory T cell [68]. Therefore, inactivating Cyclin D–CDK4/6 complex may weaken or abrogate acquired drug resistance when combined with ICB, which needs further experiments to verify.
In summary, CDK4/6i have synergistical effects with ICB that occur through multiple pathways, including the promotion of immune regulation via CDK4/6 and the reduction or elimination of resistance to ICB, indicating excellent research value and providing good combination therapy prospects (Fig. 4).
CDK4/6 inhibitors augment the sensitivity of ICB. Targeting CDK4/6 abrogates the resistance of anti-PD-L1 mAb by down-regulating PD-L1 expression through phosphorylating Rb and regulating B2M expression. It also enhances the sensitivity by down-regulating PD-L1 related to the NF-κB pathway and phosphorylating SPOP. And facilitating the occurrence of DDR, which mTOR regulates
5 Conclusion and perspectives
Although CDK4/6i promote the immune response, it remains to be further elucidated. Notably, apoptosis of tumor cells is a critical factor contributing to immune evasion. Rb has at least 16 CDK phosphorylation sites, the effects on apoptosis vary when stimulated or phosphorylated at different locations, and this may be associated with the Fas/FasL system activated by TNF. Hence, further investigation is warranted to determine whether CDK4/6i has disparate effects on Rb phosphorylation sites that impact tumor cell apoptosis and prevent immune evasion.
CDK4/6i are majorly accredited in breast cancer, the clinical feasibility in other cancer types need to be further tested. The intricate pathway crosstalk related research provide theoretical basis for CDK4/6i combination therapy. Particularly in solid malignant tumors, preoperative concurrent chemoradiotherapy and immunotherapy are chosen by more patients. CDK4/6i as radio-sensitizer has been well demonstrated in ER+ BC, HNSCC (NCT02499120), and Lung squamous cell carcinoma (LSCC) [92, 93]. Trilaciclib (G1T28) is a short-acting therapeutic agent designed specifically to protect hematopoietic stem cells from DNA-damaging chemotherapeutic agents that has been widely used in chemotherapy (NCT02499770, NCT02978716) [94, 95]. Moreover, G1T28 showed good pharmacological effect in healthy volunteers and could temporarily inhibit the proliferation of bone marrow hematopoietic stem cells and progenitor cells (HSPC) [96]. Examining drug effect and general toxicity profiles will be a critical first step. The results of these trials are eagerly awaited.
Our review demonstrates that CDK4/6i have wider applications in immunotherapy beyond conventional antitumor treatments, providing a rationale and novel therapeutic strategy for the implementation of target–immune combinations. Despite many clinical trials supporting the feasibility of target–immune combination therapy, there are still complications. In both early and advanced HR+ BC, the combination therapy resulted in a treatment-related adverse events (AEs) of hepatitis and pneumonitis, which likely caused by proinflammatory cytokine production and suppression of Treg proliferation [97]. Surprisingly, the application of CDK4/6i before surgery to create a reservoir of memory CD8+ T cells using ICB may subsequently alleviate the synergistic toxicity derived from simultaneous application. It is expected as adjuvant immunotherapy to reduce local tumor progression efficaciously, improve patient survival and prognosis and has maximal efficacy in the neoadjuvant therapy of a variety of potentially operable tumors. Thus, further investigation is warranted to optimize the therapeutic schedule of CDK4/6-based combination immunotherapy while minimizing potential complications.
Availability of data and material
The authors declare that the results reported in this manuscript are obtained from our original summary, have not been previously published, and have not been submitted for publication elsewhere.
References
Liu J, Peng Y, Wei W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022;32(1):30–44.
Suski JM, et al. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39(6):759–78.
Leal-Esteban LC, Fajas L. Cell cycle regulators in cancer cell metabolism. Biochim Biophys Acta Mol Basis Dis. 2020;1866(5): 165715.
Gao X, Leone GW, Wang H. Cyclin D-CDK4/6 functions in cancer. Adv Cancer Res. 2020;148:147–69.
Fassl A, Geng Y, Sicinski P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science. 2022;375(6577):eabc1495.
Chaudhary S, et al. Dual blockade of EGFR and CDK4/6 delays head and neck squamous cell carcinoma progression by inducing metabolic rewiring. Cancer Lett. 2021;510:79–92.
Pandey K, et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int J Cancer. 2019;145(5):1179–88.
Zhang J, et al. Mechanisms and Implications of CDK4/6 Inhibitors for the Treatment of NSCLC. Front Oncol. 2021;11: 676041.
Budimir N, et al. Reversing T-cell Exhaustion in Cancer: Lessons Learned from PD-1/PD-L1 Immune Checkpoint Blockade. Cancer Immunol Res. 2022;10(2):146–53.
Yao H, et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat Biomed Eng. 2019;3(4):306–17.
Wang Q, Qin Y, Li B. CD8(+) T cell exhaustion and cancer immunotherapy. Cancer Lett. 2023;559: 216043.
Bai XF, et al. Homotypic Targeted Photosensitive Nanointerferer for Tumor Cell Cycle Arrest to Boost Tumor Photoimmunotherapy. ACS Nano. 2022;16(11):18555–67.
De Luca A, et al. Pharmacokinetic drug evaluation of palbociclib for the treatment of breast cancer. Expert Opin Drug Metab Toxicol. 2018;14(9):891–900.
Piezzo M, et al. Targeting Cell Cycle in Breast Cancer: CDK4/6 Inhibitors. Int J Mol Sci. 2020;21(18):6479.
Goel S, et al. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018;28(11):911–25.
Du Q, et al. The application and prospect of CDK4/6 inhibitors in malignant solid tumors. J Hematol Oncol. 2020;13(1):41.
Yao Y, et al. HOXB9 blocks cell cycle progression to inhibit pancreatic cancer cell proliferation through the DNMT1/RBL2/c-Myc axis. Cancer Lett. 2022;533: 215595.
Zhou Y, et al. Wnt signaling pathway in cancer immunotherapy. Cancer Lett. 2022;525:84–96.
Ma J, et al. MYC induces CDK4/6 inhibitors resistance by promoting pRB1 degradation. Nat Commun. 2024;15(1):1871.
Zhang M, Kim S, Yang HW. Non-canonical pathway for Rb inactivation and external signaling coordinate cell-cycle entry without CDK4/6 activity. Nat Commun. 2023;14(1):7847.
Zhu X, et al. Efficacy and mechanism of the combination of PARP and CDK4/6 inhibitors in the treatment of triple-negative breast cancer. J Exp Clin Cancer Res. 2021;40(1):122.
Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5(1):60.
Crozier L, et al. CDK4/6 inhibitors induce replication stress to cause long-term cell cycle withdrawal. Embo j. 2022;41(6): e108599.
Sager RA, et al. Therapeutic potential of CDK4/6 inhibitors in renal cell carcinoma. Nat Rev Urol. 2022;19(5):305–20.
Moskovits N, et al. Palbociclib in combination with sunitinib exerts a synergistic anti-cancer effect in patient-derived xenograft models of various human cancers types. Cancer Lett. 2022;536: 215665.
Cai Z, et al. Overexpressed Cyclin D1 and CDK4 proteins are responsible for the resistance to CDK4/6 inhibitor in breast cancer that can be reversed by PI3K/mTOR inhibitors. Sci China Life Sci. 2023;66(1):94–109.
Zhang K, et al. CDK4/6 inhibitor palbociclib enhances the effect of pyrotinib in HER2-positive breast cancer. Cancer Lett. 2019;447:130–40.
Kohlmeyer JL, et al. CDK4/6-MEK Inhibition in MPNSTs Causes Plasma Cell Infiltration, Sensitization to PD-L1 Blockade, and Tumor Regression. Clin Cancer Res. 2023;29(17):3484–97.
Liu C, et al. AZD5153 reverses palbociclib resistance in ovarian cancer by inhibiting cell cycle-related proteins and the MAPK/PI3K-AKT pathway. Cancer Lett. 2022;528:31–44.
Agostinetto E, et al. CDK4/6 and PI3K inhibitors: A new promise for patients with HER2-positive breast cancer. Eur J Clin Invest. 2021;51(7): e13535.
Chen W, et al. Applications and mechanisms of the cyclin-dependent kinase 4/6 inhibitor, PD-0332991, in solid tumors. Cell Oncol (Dordr). 2022;45(6):1053–71.
Bilotta MT, Antignani A, Fitzgerald DJ. Managing the TME to improve the efficacy of cancer therapy. Front Immunol. 2022;13: 954992.
Mao X, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131.
Goel S, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–5.
Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298–312.
Pishesha N, Harmand TJ, Ploegh HL. A guide to antigen processing and presentation. Nat Rev Immunol. 2022;22(12):751–64.
Dhatchinamoorthy K, Colbert JD, Rock KL. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol. 2021;12: 636568.
Axelrod ML, et al. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin Cancer Res. 2019;25(8):2392–402.
Stopfer LE, et al. Multiplexed relative and absolute quantitative immunopeptidomics reveals MHC I repertoire alterations induced by CDK4/6 inhibition. Nat Commun. 2020;11(1):2760.
Lau PKH, et al. Enhancing Adoptive Cell Transfer with Combination BRAF-MEK and CDK4/6 Inhibitors in Melanoma. Cancers (Basel). 2021;13(24):6342.
Wu Y, et al. CDK4/6 inhibitors sensitize gammaherpesvirus-infected tumor cells to T-cell killing by enhancing expression of immune surface molecules. J Transl Med. 2022;20(1):217.
Lu Y, et al. Evidence for retinoblastoma protein (RB) dependent and independent IFN-gamma responses: RB coordinately rescues IFN-gamma induction of MHC class II gene transcription in noninducible breast carcinoma cells. Oncogene. 1994;9(4):1015–9.
Zhu X, Pattenden S, Bremner R. pRB is required for interferon-gamma-induction of the MHC class II abeta gene. Oncogene. 1999;18(35):4940–7.
Yao Y, et al. Novel insights into RB1 mutation. Cancer Lett. 2022;547: 215870.
Liu C, et al. The Immunological Role of CDK4/6 and Potential Mechanism Exploration in Ovarian Cancer. Front Immunol. 2021;12: 799171.
Zhang N, et al. PARP inhibitor plus radiotherapy reshapes an inflamed tumor microenvironment that sensitizes small cell lung cancer to the anti-PD-1 immunotherapy. Cancer Lett. 2022;545: 215852.
Fan H, et al. DNA damage induced by CDK4 and CDK6 blockade triggers anti-tumor immune responses through cGAS-STING pathway. Commun Biol. 2023;6(1):1041.
Goronzy JJ, Weyand CM. T-cell co-stimulatory pathways in autoimmunity. Arthritis Res Ther. 2008;10(Suppl 1):S3.
Charles A, et al. Low-dose CDK4/6 inhibitors induce presentation of pathway specific MHC ligands as potential targets for cancer immunotherapy. Oncoimmunology. 2021;10(1):1916243.
Schober SJ, et al. The Oncolytic Adenovirus XVir-N-31 Joins Forces with CDK4/6 Inhibition Augmenting Innate and Adaptive Antitumor Immunity in Ewing Sarcoma. Clin Cancer Res. 2023;29(10):1996–2011.
Kumar A, et al. Dendritic cell therapy augments antitumor immunity triggered by CDK4/6 inhibition and immune checkpoint blockade by unleashing systemic CD4 T-cell responses. J Immunother Cancer. 2023;11(5):e006019.
Cai H, et al. Defects in Macrophage Reprogramming in Cancer Therapy: The Negative Impact of PD-L1/PD-1. Front Immunol. 2021;12: 690869.
Lelliott EJ, Sheppard KE, McArthur GA. Harnessing the immunotherapeutic potential of CDK4/6 inhibitors in melanoma: is timing everything? NPJ Precis Oncol. 2022;6(1):26.
Egelston C, et al. Pre-existing effector T-cell levels and augmented myeloid cell composition denote response to CDK4/6 inhibitor palbociclib and pembrolizumab in hormone receptor-positive metastatic breast cancer. J Immunother Cancer. 2021;9(3):e002084.
Zhang QF, et al. CDK4/6 inhibition promotes immune infiltration in ovarian cancer and synergizes with PD-1 blockade in a B cell-dependent manner. Theranostics. 2020;10(23):10619–33.
Lelliott EJ, et al. CDK4/6 Inhibition Promotes Antitumor Immunity through the Induction of T-cell Memory. Cancer Discov. 2021;11(10):2582–601.
Lai AY, et al. CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy. J Immunother Cancer. 2020;8(2):e000847.
Xu R, et al. Neoantigen-targeted TCR-T cell therapy for solid tumors: How far from clinical application. Cancer Lett. 2022;546: 215840.
Deng J, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018;8(2):216–33.
Lee JU, Kim LK, Choi JM. Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases. Front Immunol. 2018;9:2747.
Wang R, et al. Lipid metabolism reprogramming of CD8(+) T cell and therapeutic implications in cancer. Cancer Lett. 2023;567: 216267.
Zheng NS, et al. CD147-specific chimeric antigen receptor T cells effectively inhibit T cell acute lymphoblastic leukemia. Cancer Lett. 2022;542: 215762.
Veiga-Fernandes H, Rocha B. High expression of active CDK6 in the cytoplasm of CD8 memory cells favors rapid division. Nat Immunol. 2004;5(1):31–7.
Heckler M, et al. Inhibition of CDK4/6 Promotes CD8 T-cell Memory Formation. Cancer Discov. 2021;11(10):2564–81.
Scirocchi F, et al. Immune effects of CDK4/6 inhibitors in patients with HR(+)/HER2(-) metastatic breast cancer: Relief from immunosuppression is associated with clinical response. EBioMedicine. 2022;79: 104010.
Peuker CA, et al. Treatment with ribociclib shows favourable immunomodulatory effects in patients with hormone receptor-positive breast cancer-findings from the RIBECCA trial. Eur J Cancer. 2022;162:45–55.
Xu L, et al. The IL-33-ST2-MyD88 axis promotes regulatory T cell proliferation in the murine liver. Eur J Immunol. 2018;48(8):1302–7.
Liu J, et al. CCND1 Amplification Profiling Identifies a Subtype of Melanoma Associated With Poor Survival and an Immunosuppressive Tumor Microenvironment. Front Immunol. 2022;13: 725679.
Blakely B, Shin S, Jin K. Overview of the therapeutic strategies for ER positive breast cancer. Biochem Pharmacol. 2023;212: 115552.
Li P, et al. Anti-IL-8 antibody activates myeloid cells and potentiates the anti-tumor activity of anti-PD-1 antibody in the humanized pancreatic cancer murine model. Cancer Lett. 2022;539: 215722.
Vilgelm AE, et al. Connecting the Dots: Therapy-Induced Senescence and a Tumor-Suppressive Immune Microenvironment. J Natl Cancer Inst. 2016;108(6):djv406.
Dowless M, et al. Abemaciclib Is Active in Preclinical Models of Ewing Sarcoma via Multipronged Regulation of Cell Cycle, DNA Methylation, and Interferon Pathway Signaling. Clin Cancer Res. 2018;24(23):6028–39.
Hosoya T, et al. Cell cycle regulation therapy combined with cytokine blockade enhances antiarthritic effects without increasing immune suppression. Ann Rheum Dis. 2016;75(1):253–9.
Schaer DA, et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell Rep. 2018;22(11):2978–94.
Lee DH, et al. CDK4/6 inhibitors induce breast cancer senescence with enhanced anti-tumor immunogenic properties compared with DNA-damaging agents. Mol Oncol. 2024;18(1):216–32.
Chen K, et al. Single cell RNA-seq reveals the CCL5/SDC1 receptor-ligand interaction between T cells and tumor cells in pancreatic cancer. Cancer Lett. 2022;545: 215834.
Uzhachenko RV, et al. Metabolic modulation by CDK4/6 inhibitor promotes chemokine-mediated recruitment of T cells into mammary tumors. Cell Rep. 2021;35(1): 108944.
Wang J, et al. Metabolism and polarization regulation of macrophages in the tumor microenvironment. Cancer Lett. 2022;543: 215766.
Thomas JK, et al. CC chemokines are differentially expressed in Breast Cancer and are associated with disparity in overall survival. Sci Rep. 2019;9(1):4014.
Jin X, et al. Phosphorylated RB Promotes Cancer Immunity by Inhibiting NF-κB Activation and PD-L1 Expression. Mol Cell. 2019;73(1):22-35.e6.
Ge S, et al. Molecular imaging of immune checkpoints in oncology: Current and future applications. Cancer Lett. 2022;548: 215896.
Garutti M, et al. CDK4/6 Inhibitors in Melanoma: A Comprehensive Review. Cells. 2021;10(6):1334.
Jang HJ, et al. Inhibition of Cyclin Dependent Kinase 4/6 Overcomes Primary Resistance to Programmed Cell Death 1 Blockade in Malignant Mesothelioma. Ann Thorac Surg. 2022;114(5):1842–52.
Antonangeli F, et al. Regulation of PD-L1 Expression by NF-κB in Cancer. Front Immunol. 2020;11: 584626.
Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24.
Zeng X, et al. Down-regulation of RCC1 sensitizes immunotherapy by up-regulating PD-L1 via p27(kip1) /CDK4 axis in non-small cell lung cancer. J Cell Mol Med. 2021;25(8):4136–47.
Yu J, et al. Genetic Aberrations in the CDK4 Pathway Are Associated with Innate Resistance to PD-1 Blockade in Chinese Patients with Non-Cutaneous Melanoma. Clin Cancer Res. 2019;25(21):6511–23.
Wang H, Liu B, Wei J. Beta2-microglobulin(B2M) in cancer immunotherapies: Biological function, resistance and remedy. Cancer Lett. 2021;517:96–104.
Zhang T, et al. Addressing resistance to PD-1/PD-(L)1 pathway inhibition: considerations for combinatorial clinical trial designs. J Immunother Cancer. 2023;11(5):e006555.
Pathak R, et al. Acquired Resistance to PD-1/PD-L1 Blockade in Lung Cancer: Mechanisms and Patterns of Failure. Cancers (Basel). 2020;12(12):3851.
Ribas A, et al. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol Res. 2016;4(3):194–203.
Yang Y, et al. CDK4/6 inhibitors: a novel strategy for tumor radiosensitization. J Exp Clin Cancer Res. 2020;39(1):188.
Satpathy S, et al. A proteogenomic portrait of lung squamous cell carcinoma. Cell. 2021;184(16):4348-4371.e40.
Teh JLF, Aplin AE. Arrested Developments: CDK4/6 Inhibitor Resistance and Alterations in the Tumor Immune Microenvironment. Clin Cancer Res. 2019;25(3):921–7.
Bisi JE, et al. Preclinical Characterization of G1T28: A Novel CDK4/6 Inhibitor for Reduction of Chemotherapy-Induced Myelosuppression. Mol Cancer Ther. 2016;15(5):783–93.
He S, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9(387):eaal3986.
Masuda J, et al. Efficacy, safety, and biomarker analysis of nivolumab in combination with abemaciclib plus endocrine therapy in patients with HR-positive HER2-negative metastatic breast cancer: a phase II study (WJOG11418B NEWFLAME trial). J Immunother Cancer. 2023;11(9):e007126.
Klein ME, et al. CDK4/6 Inhibitors: The Mechanism of Action May Not Be as Simple as Once Thought. Cancer Cell. 2018;34(1):9–20.
Michel L, et al. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016;58:41–8.
Braal CL, et al. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs. 2021;81(3):317–31.
Dhillon S. Trilaciclib: First Approval. Drugs. 2021;81(7):867–74.
Zhang P, et al. A phase 1 study of dalpiciclib, a cyclin-dependent kinase 4/6 inhibitor in Chinese patients with advanced breast cancer. Biomark Res. 2021;9(1):24.
Andreano KJ, et al. G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer. Breast Cancer Res Treat. 2020;180(3):635–46.
Dennis MJ, et al. A phase I study of avelumab, palbociclib, and cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma. Oral Oncol. 2022;135: 106219.
Solomon B, et al. A WIN Consortium phase I study exploring avelumab, palbociclib, and axitinib in advanced non-small cell lung cancer. Cancer Med. 2022;11(14):2790–800.
Wang S, et al. Platform study of genotyping-guided precision medicine for rare solid tumours: a study protocol for a phase II, non-randomised, 18-month, open-label, multiarm, single-centre clinical trial testing the safety and efficacy of multiple Chinese-approved targeted drugs and PD-1 inhibitors in the treatment of metastatic rare tumours. BMJ Open. 2021;11(6): e044543.
Middleton G, et al. The National Lung Matrix Trial: translating the biology of stratification in advanced non-small-cell lung cancer. Ann Oncol. 2015;26(12):2464–9.
Italiano A, et al. Molecular profiling of advanced soft-tissue sarcomas: the MULTISARC randomized trial. BMC Cancer. 2021;21(1):1180.
Kreutzfeldt J, et al. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res. 2020;10(4):1045–67.
Patnaik A, et al. Safety and Clinical Activity of a New Anti-PD-L1 Antibody as Monotherapy or Combined with Targeted Therapy in Advanced Solid Tumors: The PACT Phase Ia/Ib Trial. Clin Cancer Res. 2021;27(5):1267–77.
Chang H, et al. Prognostic Value of CD200R1 mRNA Expression in Head and Neck Squamous Cell Carcinoma. Cancers (Basel). 2020;12(7):1777.
Pujol JL, et al. Abemaciclib in Combination With Pembrolizumab for Stage IV KRAS-Mutant or Squamous NSCLC: A Phase 1b Study. JTO Clin Res Rep. 2021;2(11): 100234.
Fennell DA, et al. Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): an open-label, single-arm, phase 2a clinical trial. Lancet Respir Med. 2021;9(6):593–600.
Chen H, et al. Tumor Cell-Autonomous SHP2 Contributes to Immune Suppression in Metastatic Breast Cancer. Cancer Res Commun. 2022;2(10):1104–18.
Daniel D, et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: A multicentre, randomised, double-blind, placebo-controlled Phase II trial. Int J Cancer. 2021;148(10):2557–70.
Chen MK. Efficacy of PARP inhibition combined with EZH2 inhibition depends on BRCA mutation status and microenvironment in breast cancer. FEBS J. 2021;288(9):2884–7.
Funding
This work was supported by the China National Natural Scientific Fund No. 82073010 (to X.Z.), No. 82172764 (to Y.R.) and No. 82002892 (to Y.W.).
Author information
Authors and Affiliations
Contributions
X.Z., Y.R., and M.S. designed framework. M.S., L.D., C.L., J.D., B.W., and B.X. collected the data. M.S., L.D., Y.W., C.L., X.Y., Y.R., and X.Z. wrote and edited the manuscript. X.Z., Y.R., and Y.W. provided guidance and technical support.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Each of the authors has read and abided by the statement of ethical standards for manuscripts submitted to Holistic Integrative Oncology.
Consent for publication
Each of the authors has contributed to, read, and approved the final version of this manuscript, and the order of the authors has been agreed upon by all authors.
Competing interests
All authors claim that there is no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, M., Dong, L., Wang, Y. et al. The role of targeting CDK4/6 in cancer immunotherapy. Holist Integ Oncol 3, 32 (2024). https://doi.org/10.1007/s44178-024-00100-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00100-0