Abstract
The outbreak of COVID-19 has drastically affected the daily lifestyles of people globally where specific Coronavirus-2 transmits primarily by respiratory droplets. Structurally, the SARS-CoV-2 virus is made up of four types of proteins in which S-protein is indispensable among them, as it causes rapid replication in the host body. Therefore, the glycine and alanine composed of HR1 of S-protein is the ideal target for antiviral action. Different forms of surface-active PPEs can efficiently prevent this transmission in this circumstance. However, the virus can survive on the conventional PPEs for a long time. Hence, the nanotechnological approaches based on engineered nanomaterials coating on medical equipments can potentially prevent the dissemination of infections in public. Silver nanoparticles with tuneable physicochemical properties and versatile chemical functionalization provide an excellent platform to combat the disease. The coating of amine-functionalized silver nanoparticle (especially amine linked to aliphatic chain and trialkoxysilane) in its nanostructured form enables cloths trap and kill efficient. PPEs are a primary and reliable preventive measure, although they are not 100% effective against viral infections. So, developing and commercializing surface-active PPEs with trap and kill efficacy is highly needed to cope with current and future viral infections. This review article discusses the COVID-19 morphology, antiviral mechanism of Ag-NPs against SARS-CoV-2 virus, surface factors that influence viral persistence on fomites, the necessity of antiviral PPEs, and the potential application of amine-functionalized silver nanoparticles as a coating material for the development of trap and kill-efficient face masks and PPE kits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A novel coronavirus (SARS-CoV-2) causative agent of COVID-19 was identified in December 2019 in China. COVID-19 was declared a pandemic by World Health Organization declared on March 11, 2020 [1]. As of Feb.11, 2022, 404,910,528 confirmed cases of COVID-19, including 5,783,776 deaths as reported by WHO [2]. However, limiting the transmission of COVID-19 infection in public is still a big challenge for researchers and clinicians. To date, nanotechnological approaches for diagnosing and treating diseases are indispensable in the scientific and medical fields [3, 4]. A new hope has emerged for the medical field by functionalizing nanosized materials, which provides a wide range of methodologies that allow researchers to manipulate the structural and functional properties [5, 6]. The current emerging medical challenge lies in developing personal protective equipment that is reusable, long durable, and capable of inactivating viral and bacterial pathogens, thus, reducing the risk of infection and transmission. Most functional antimicrobial coatings developed are antibacterial, but very few are commercialized as antiviral. Hence, it is highly needed to develop potential antiviral and viricidal coatings that can trap and kill viruses on personal protective equipment (PPE), hygienic implements, and other devices to fight against this viral pandemic [7]. Materials for antiviral coatings have been classified into three major groups, i.e. antiviral polymers, metal ions or oxides, and surface-functionalized nanoparticles, based on the used materials at the contaminated sites [8].
Metal nanoparticles are well-known antiviral agents that are used with other disinfectants [9]. Among all metal nanomaterials, silver nanoparticles (Ag-NPs) are broad-spectrum antimicrobial agents, thus, the most widely commercialized nanomaterials [10, 11]. Silver ions and nanoparticles have proven to be a potent inactivators of human pathogenic bacteria, fungi, and viruses. Biocidal activity against leading bacterial and fungal pathogens, including COVID-19-associated mucormycosis (CAM), methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa, Vibrio cholera, and Bacillus subtilis has been evaluated and reported [12,13,14,15,16,17,18]. Similarly, synergistic antimicrobial activity of silver nanoparticles against S. aureus, E. coli, Salmonella typhi, and Micrococcus luteus with various antibiotics such as ampicillin, penicillin G, amoxicillin, kanamycin, erythromycin, clindamycin, chloramphenicol, and vancomycin was reported [19,20,21]. However, due to the reduced size of starting bulk materials, nanoparticles tend to achieve higher surface area and energy, making them unstable [22]. Hence, stabilizing nanoparticles from agglomeration often requires capping or stabilizing agents. Capping agents form a layer around nanoparticle through different mechanisms depending on the material and net charge of the polymer. These forces can be categorized as steric stabilization, electrostatic interactions, depletion stabilization, stabilization by hydration forces, and Van der Waals forces [23]. Some capping agents stabilize nanoparticles through multiple mechanisms, such as branched polyethyleneimine (BPEI). BPEI stabilizes Ag-NPs electrostatically and sterically due to BPEI’s charge and branched and flexible structure [24]. Polymers are chemical compounds with molecules bonded together in long and repeating chains. Polymers represent excellent characteristics such as ductility, resistance, and elasticity, making polymers a versatile material for biomedical applications. Various cationic and anionic polymers are currently applied to synthesize nanomaterials as reducing, stabilizing, and capping agents. Cationic polymer-functionalized silver nanoparticles have a soft edge of being an excellent antiviral agent. Structurally, viruses are made up of proteins and nucleic acid. The genome of CoVs (27–32 kb) is a single-stranded positive-sense RNA (+ssRNA) more extensive than any other RNA viruses. The nucleocapsid protein (N) forms the capsid outside the genome, and the genome is further backed by an envelope that is associated with three structural proteins: membrane protein (M), spike protein (S), and envelope protein (E) [25, 26]. Silver nanoparticle interacts very strongly with thiol (-SH) and -COOH groups in protein that perturb the virus's surface and ultimately inactivate. Most viruses infect their human host via inhalation of microdroplets that float in the air, especially SARS-CoV-2 causing COVID-19. A cotton mask or respirator could be used to prevent the entrance of the virus into the respiratory tract. However, the masks cannot kill the SARS-CoV-2 virus outside the host. To make cotton masks, it needed to activate the mask with trap and kill functionality by coating it with cationic polymers-functionalized silver nanoparticles. This review article aims to overview previous and recent approaches to polymer-functionalized silver nanoparticle-based antiviral coatings, especially against the coronavirus, and focuses on possible strategies to fight against the current and possible future pandemic.
Factors Influencing the Long-Term Virus Persistence on Fomites and Necessity of Antiviral Surface Coatings
Dissemination of the viruses between humans is generally caused by an infected individual's respiratory, skin, or other bodily fluid secretions. The viruses can be transmitted directly or indirectly through aerosols, droplets, or contact with contaminated fomites. Viral contamination of surfaces could cause serious infections within a community. For example, norovirus (a non-enveloped virus) could persist for several weeks on different fomites, resulting in lethal outbreaks in healthcare facilities [27]. Therefore, understanding how long viruses can persist on a surface can help control strategies and medical authorities to control infection outbreaks. Fundamentally four main properties can affect the persistence of viruses on surfaces (Fig. 1). These include (i) porosity of material; (ii) physical properties of material surface such as relative humidity, temperature, exposure to light, and surface roughness; (iii) biological properties, like the structure of the adsorbing virus or prior presence of other microorganisms; and (iv) chemical properties of surfaces such as pH, the presence of reactive ions, adsorption state, and organic matter. The surrounding organic material, such as saliva or mucus droplets, can stabilize and protect many viruses. In addition, it has been reported that substances such as bacteria, fats, and proteins in the viral inoculum can increase persistence [28,29,30,31]. Major contributing factors concerning surface properties include, among others, porosity [27], absorption [32], and surface hydrophobicity [33]. Each type of virus has unique proteins that interact with a surface uniquely, so designing an effective antiviral surface coating may need to be designed for a specific type of virus.
Surface properties influence the persistence of viruses. These include physical properties (including light exposure, temperature, and humidity), chemical properties (such as pH or antiviral coatings), and biological properties (depending on the virus vulnerabilities, e.g. an envelope) as well as the type of surfaces such as the porosity or topography. Adopted as ACS COVID-19 subset from [31]
The porosity of abiotic surfaces plays a significant role in viral adsorption and survival; hence, several reports have compared the persistence and time of complete decay of different viruses on porous and non-porous surfaces [34,35,36]. It is found that most viruses exhibit greater persistence on non-porous materials than on porous surfaces [27, 35], although there are exceptions also exist [37, 38]. For example, influenza virus A can persist more than 24–28 h on stainless steel and plastic surfaces at 35–40% humidity. Nevertheless, infectious viral particles dropped to undetectable levels on porous surfaces such as cloth or paper after 8–12 h [37, 39]. The poor persistence of viral particles on porous surfaces could be due to the complete drying of the viruses on the surfaces or low elution of the viruses from these materials [35]. It is observed that both the SARS-CoV-1 and SARS-CoV-2 coronavirus have similar persistence in terms of surface porosity [36]. It has been reported SARS-CoV-2 virus remains infectious even after seven days on a surgical mask, whereas comparatively, no viable virus was detected on the surface of stainless steel and plastic [40].
Several studies indicated that the antiviral properties of fomites could be linked to the ability of a surface to absorb a virus [32]. Due to the nature of liquid absorption property of absorbent materials such as cotton and cardboard could offer strong protection against virus-bearing respiratory droplets and aerosols [41, 42]. For example, Lai and co-workers compared the survival of SARS-CoV-2 between two forms of PPE gowns, a hydrophobic disposable type and a cotton gown [43]. They observed that the cotton material has significant potential to absorb highly concentrated droplets of viral particles, and no viable virus was detected after 1 h, whereas in the case of disposable gowns, most of the virus remained viable for up to 24 h. Therefore, an outer fluid-absorbing layer in PPE garments and medical devices may be advantageous.
Viral persistence can also be affected by surface hydrophobicity of material surface and outer layer of virus capsids [33]. Hence, the hydrophobicity of the outer layer of proteins in virus capsids can significantly influence their interactions with solid surfaces and the surrounding environment [44]. Therefore, building a fundamental understanding of the interactions between viruses, i.e. the outer surface of proteins and solid surfaces, is vital for controlling environmental transmission and designing efficient antiviral strategies. Both experimental and computational analyses have been used to determine the hydrophobicity of different viruses [45, 46]. For example, Chattopadhyay et al. demonstrated that the sorption of hydrophobic viruses, i.e. viruses carrying hydrophobic proteins' outer layers, was favoured by surfaces coated with hydrophobic sorbents, while the sorption of hydrophilic viruses was favoured by hydrophilic surfaces [47].
The Application Rationality and Previously Assessed Antiviral Potential of Silver Nanoparticles
For hundreds of years, silver has been used as an antimicrobial agent against a broad spectrum of pathogenic microorganisms in the form of "Rajatbhasma" (an Ayurvedic medicine prepared from silver) [48, 49]. Before the commercialization of antibiotics, silver was used as an antiseptic to treat open wounds and burns. Silver nanoparticles demonstrated strong antiviral potential against several viruses, which include the following families: retroviridae, hepadnaviridae, paramyxoviridae, herpesviridae, poxviridae, orthomyxoviridae, and Arenaviridae [50]. Silver nanoparticles showed potential antiviral activities against a broad range of viruses. In addition, viruses are less likely to become resistant to silver nanoparticles than conventional antiviral agents. The nanoparticles have multivalent interactions with viral surface components and cell membrane receptors which block viral entry into the cells [51]. As an antiviral agent, silver nanoparticles can act directly and rapidly on viral particles, bind with virus coat proteins, and disrupt structure before binding to the host cell. An interesting study has recently been conducted where 25-nm Ag-NPs could mediate a consistent reduction in Vaccinia virus (VACV) entry at non-cytotoxic concentrations. Ag-NPs prevented direct fusion and macropinocytosis-dependent entry of VACV; cells where a vital component of macropinocytosis (Pak1) had been knocked down, showed a reduced loss of Ag-NP anti-entry effects. Furthermore, Western blot analysis suggested that Ag-NPs bind directly to the entry fusion complex of VACV, revealing a potential virucidal mechanism [52]. Silver nanoparticle interaction with viral biomolecules suggests that silver nanoparticles have a huge potential to face the challenge of viral infections and enhance the quality of existing antiviral therapies. Depending on the interaction and virucidal effect of silver nanoparticles against viruses such as hepatitis B virus [53], HIV-1 [54, 55], herpes simplex virus type 1 [56], respiratory syncytial virus [57], tacaribe virus [58], monkeypox virus [59], and influenza virus [60, 61], it can be predicted that silver nanoparticles act as protective antiviral shields. The toxicity of silver nanoparticles against viruses has been reported by Sinclair et al. to be dependent on several physicochemical factors such as particle concentration, size, and shape [62]. The results demonstrated that surface charge was a crucial factor governing their antiviral activity [62]. The researchers investigated the influence of capping agents representing various surface charges ranging from negative to positive. These Ag-NPs were capped with citrate, polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), mercaptoacetic acid (MAA) and (branched polyethyleneimine (BPEI). Among the capping agents investigated, BPEI capped Ag-NPs exhibited the highest reduction in PFU of MS2 bacteriophage (≥ 6 log10-units reductions), followed by 4–5 log10-units reductions with PVP and PEG cappings and 3–4 log10-units with MAA and citrate capping [63]. Further, an in vivo study demonstrated that Ag-NPs reduced RSV replication, both in epithelial cell lines and in experimentally infected BALB/c mice [64]. Several studies on antiviral and antimicrobial activity of colloidal silver nanoparticles against enveloped and non-enveloped viruses and a broad range of pathogenic microorganisms have proven its significant potential as an excellent nanomaterial to cope with current and unfortunate future viral and microbial-originated health crises.
A Brief Description on Amine-Functionalized Active Moieties Used for the Synthesis of Silver Nanoparticles and Its Biocidal Activity
Like other metal nanoparticles, the synthesis of silver nanoparticles can be achieved by three different processes, namely, chemical, physical, and biological synthesis. Physical synthesis requires physical forces that crush bulk materials into nanostructures. Due to the absence of capping or stabilizing agents, a high probability of agglomeration occurs. As a result, deterring it poses a significant challenge. Additionally, this synthesis requires external energy and sophisticated equipment. Synthesis of silver nanoparticles requires a robust-reducing agent and a capping agent that prevent aggregation or agglomeration of synthesized silver nanoparticles; capping or coating is an excellent way to stabilize the nanoparticles through the establishment of electrostatic, steric, or electrostatic interactions between particles. Various chemical capping agents, such as polyphenols, plant extract, surfactant, polymers, peptides, Graphene oxide (GO), Series of N-acyl tyramines, and carboxylic acid derivatives were used.
For example, citrate has been widely used as a reducing and capping agent because of its chemical properties as a pH regulator and ligand protection. As a capping agent, citrate-based ionic liquids wrap the nanoparticles, block their excessive growth, and act as a charge controller [65]. Variety of publication on synthesis and activity of silver nanoparticles have been reported in the literature [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,92,93,94,95,96,97,98,99,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,123]. Recently, the role amine in the synthesis of silver nanoparticles has been reported in details [124]. We have for the first time demonstrated the role of amine-functionalized trialkoxysilane in controlled synthesis of noble metal nanoparticles and its multimetallic analogues [67,68,69]. The role of amine in silver nanoparticles synthesis has recently been discussed in detail [124]. Apart from the role of amine-functionalized trialkoxysilanes in silver nanoparticles formation, the role of amine lined to macromolecules that bear positive charges has also been demonstrated [74]. The charge can be present intrinsically in the polymer backbone or the side chain groups. Most cationic polymers carry protonated primary, secondary, or tertiary amine functional groups. These polymers differ widely in their polymeric structure. They can be linear, branched, hyperbranched, or dendrimer and can be further differentiated by the presence of the positive charges in molecular structure, i.e. in the backbone or at the side chains. Various cationic polymers reduce and capping agents to synthesize silver nanoparticles. These polymers can be natural, semisynthetic, or synthetic polymers such as gelatine, chitosan, dextran, dextrin, branched polyethyleneimine (BPEI), linear polyethyleneimine (LPEI), poly-l-lysine, etc. [66]. Luo et al. reported that gelatine capping could avoid the agglomeration of silver nanoparticles due to the amino and carboxylic groups of gelatine aligning with the surface of silver atoms in the Ag-NP, further improving stability [70]. In this section, we are discussing only polyethyleneimine and chitosan-functionalized silver nanoparticles. Polyethyleneimine (PEI) is the most outstanding example of synthetic polymer because of its wide range of commercial and biomedical applications. It can be in linear (LPEI) as well as in branched (BPEI) structures [71,72,73]. Linear PEI possesses primary and secondary amino groups, whereas BPEI also features tertiary amino groups.
BPEI usually has a ratio of primary to secondary to tertiary amino functionalities of 1:2:1, and up to 25% of these amino groups are protonated under physiological conditions. Such buffer capability can also be utilized for endosomal escape mechanisms. Polyethyleneimines are most studied due to their excellent capping and stabilizing ability for silver and gold nanoparticles [74, 75]. Tiwari et al. [74] applied microwave and an organic-reducing agent (cyclohexanone and formaldehyde) for rapid reduction of silver cations (Ag+− Ag0) along with 1-vinyl 2 pyrrolidone (VPP) and three different molecular weights of polyethyleneimines (PEI) as capping and stabilizing agent. The synthesized Ag-NPs showed considerable changes in size and stability as a function of Mw of PEI.
Furthermore, it was found that the molecular weight of PEI controls the size of nanoparticles. The higher molecular weight of PEI leads to the synthesis of larger sizes (~ 20 nm) of NPs than lower molecular weights [74]. In another approach, Zhiguo et al. prepared PEI-functionalized silver nanoparticles through the single-step hydrothermal method without any reducing agent. In this preparation, PEI acted as both stabilizing and reducing agents with excellent stability and antibacterial activity [76]. Further, in a one-pot, size-controlled preparation approach, Kim et al. [77] synthesized PEI-functionalized silver nanoparticles in an aqueous solution and assembled a 2D film at a toluene water interface. The prepared 2D film was applied for the antibacterial coating of fabrics in gauze [77].
Chitosan (CS) is one of the most popular polycationic polymers for producing intelligent nanoparticles [78]. CS had many superior functions such as antimicrobial, antioxidant, and antitumor activities, low allergenicity, high biocompatibility, and biodegradability, and non-toxicity, making it an effective functional compound in the antimicrobial agent sector [79, 80]. For example, CS can attach to metal nanoparticles to modify their antimicrobial functions, such as CS-silver nanoparticles, in which Ag+ links with nitrogen or oxygen on the CS backbone. It was shown that surface-functionalized Ag nanoparticles via CS improved antimicrobial activity against E. coli and S. aureus [81]. In a straightforward and rapid green chemistry-based method, Shahid-ul-Islam et al. fabricated chitosan-based silver nanoparticles onto linen fabric in pineapple crown extract biomolecules such as sucrose, fructose, and glucose [82]. Besides the above-discussed cationic amine polymer-functionalized silver nanoparticles, various cationic silver nanoparticles are synthesized and evaluated for their antiviral and antimicrobial activity. However, very few are incorporated in antiviral applications such as antiviral coating of PPE kit, surface coating, and air-filtration units. Hence, functionalized silver nanoparticles have shown enormous potential as a coating material and need an interdisciplinary approach to developing and commercializing multifunctional biomedical devices.
Molecular Mechanism of Functional Silver Nanoparticle-Mediated Inactivation of Viruses
Metal nanoparticles and oxides have been widely explored for antiviral therapy over the last few decades with the development of surface functionalization strategies [83]. In recent studies, Ag, Au, TiO2, SiO2, CeO2, and CuCl2 nanoparticles have been evaluated for their antiviral activity against different viruses, including hepatitis B virus (HBV), H3N2 and H1N1, HIV-1, herpes simplex virus (HSV), vesicular stomatitis, foot-and-mouth disease, and dengue virus type-2 [55, 84,85,86,87,88,89,90,91].
Silver nanoparticles (Ag-NPs) have been explored and applied as a therapeutic drug because of their unique physiochemical and physicochemical properties such as anti-inflammatory, anti-angiogenesis, antiplatelet, antifungal, anticancer, and antibacterial activities [92]. In addition, ag-NPs have gained increasing attention in the biological and medical fields due to their easy synthesis process. Further, Ag-NPs have been employed as biomedical therapeutic agents, such as wound dressings, burn care products, and silver nanoparticles containing antibacterial lotions are commercially available. However, silver nanoparticle-based antiviral agents and devices are still in the primary stage, and very few studies have been published [93, 94].
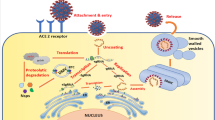
Viral entry in host cells is necessary in order to multiply. The viral infection is a multistep process that involves attachment via selective receptor expressed on the host cell surface, penetration/internalization of virion through endocytosis, uncoating, replication, assembly, and release of virions through exocytosis. At the first step to infect the host, viruses interact through specific receptors on the host cell membrane via ligand proteins in the viral glycoproteins, which could be embedded in the viral envelope. The interactions' specificity depends on the type of virus that infects the host cells. Primarily, SARS-CoV-2 initially binds to the host-receptor cell at the angiotensin-converting enzyme 2 (ACE-2) protein site on bronchial epithelial cells. The binding of SARS-CoV-2 with ACE-2 receptors of cells leads to membrane fusion with subsequent release of the viral genetic material into the host cytoplasm. The released viral RNA from SARS-CoV-2 transcribes into the viral mRNA by hijacking the host cell machinery, directing viral component protein synthesis. Finally, the synthesized viral proteins and genetic material assemble into new virions inside the host cell, subsequently released into neighbouring cells via exocytosis (Fig. 2) [95, 96]. A multistep process for establishing infection leads researchers to modulate and inactivate them before or after infection.
SARS-CoV-2 structure and pathophysiology. A SARS-CoV-2 life cycle: The viral spike (S) protein binds to the ACE-2 receptor of the host. Following the entry, the virus envelope’s proteolytic cleavage occurs and facilitates the release of viral genomic RNA in the cytoplasm, forming small RNAs (subgenomic mRNAs). These mRNAs are translated to several viral proteins (i.e. S, M, N, etc.) essential for the reassembly of the virus particle. These synthesized proteins (S, E, and M) enter the endoplasmic reticulum (ER), where nucleoprotein complex formation occurs from the combination of the nucleocapsid (N) protein and genomic-positive RNA strand. The complete functional virus particle (proteins and viral genomic RNA assembly) occurs in the ER-Golgi compartments of the host cell. These virus particles are then transported and released via vesicle formation and exocytosis. Adopted as ACS COVID-19 subset from [96]
Silver nanoparticles target and block virus attachment, penetration, replication, and budding. Functionalized silver nanoparticles interact and inactivate the virus directly or indirectly and prevent the attachment to host cells with subsequent blocking of viral replication [97]. Most often, silver nanoparticles block the interaction of viral spike protein (S) to the host cell ACE-2 receptor by altering the structure of the capsid protein and eventually reducing virulence (Fig. 3) [98]. For example, Lara et al. [55] reported that Ag-NPs bind to glycoprotein gp120 of the HIV envelope, preventing CD4-dependent virion binding, fusion, and infectivity in cell-free and cell-associated viral assays [55]. Recently, Cagno et al. demonstrated the antiviral mechanisms of broad-spectrum, non-toxic nanoparticles against HSV, human papillomavirus, RSV, dengue, and lentivirus [99]. In a fluorescence spectroscopic approach, Tiwari et al. 2020 studied the interaction of polyethyleneimine (PEI)-functionalized silver nanoparticles with cells of Acinetobacter baumannii to understand the molecular mechanism of silver nanoparticle interaction and cell surface-expressed proteins along with the impact on cells [74]. Tiwari et al. have shown that the polyethyleneimine-functionalized silver nanoparticles selectively bind and quench the autofluorescence of surface-expressed proteins, subsequently damaging the cell structures at shallow MIC values (≈ 5 µg mL−1) [74].
Possible antiviral mechanism of nanoparticles (NPs). It was adapted with permission [98] under a common creative attribution licence
Further, they have also utilized other capping agents like 3-aminopropyletrimethoxysilane, 3-glysidoxypropyletrimthoxysilane, and organic-reducing agents such as cyclohexanone and formaldehyde [67,68,69]. However, the MIC value against the Acinetobacter bauminnii, was high compared to PEI-functionalized Ag-NPs. Mechanistically, compared to the size of the SARS-CoV-2 virus (100–150 nm), including other human and plant pathogenic viruses, the 5–10 nm sized silver nanoparticles can bind with these viruses strongly. Thus, it should be considered that SARS-CoV-2 viruses have a proteaceous spike and bind with the host cell receptors that play a fundamental role during the infection and internalization of the virus particle in the host cell. The inactivation of viral spike protein by functionalized silver nanoparticles can prevent and block the binding with host ACE-2 receptors. In another approach, the small size of selectively functionalized silver nanoparticles (5–10 nm) can enter the host cell, interfere with, and inactivate the replicating viruses. The surface charge and size of silver nanoparticles can also be tuned as per requirement by using a varying molecular weight of PEI. It has also been studied that the higher molecular weight of PEI-functionalized silver nanoparticles had a more potent effect on the bacterial cell. The polyethyleneimines are cationic and hydrophilic polymers, and the functionalization of silver nanoparticles has a great scope as an air disinfectant, antiviral surface coatings, antiviral coating of masks, PPE kits, and household exhausts as front-line defence material against COVID-19 or other viral and microbial pathogens from humans to plants [100].
Amine-Functionalized Silver Nanoparticle Activated Antiviral Face Masks
Airborne transmission of SARS-CoV-2 has been recognized as virus-containing aerosols, less than five μm in droplet size can be floating in the air for a long duration, circulating in a closed environment remains infectious and, thus, be involved in the short- and long-range transmission of airborne diseases [103,104,103]. To overcome such challenges, one accepted strategy to control SARS-CoV-2 airborne transmission is to wear face masks and respirators to prevent inhalation in the respiratory tract. However, most available cloth masks do not always have satisfactory aerosol removal efficiency, droplet repulsion, and breathability (Fig. 4) [104, 105]. Fundamentally, SARS-CoV-2 can also potentially spread through indoor heating, ventilation, and air-conditioning (HVAC) systems [106]. Unfortunately, most HVAC air filters in residential, commercial, and industrial buildings cannot capture inactive airborne viruses [107]. The COVID-19 pandemic has created a huge demand worldwide for all types of face masks and respirators. As estimated in 2019, the global face mask market was at $1,520.0 million; by 2027, it is expected to be $2,455.4 million [108, 109]. In addition, the Centre for Disease Control and Prevention (CDC) has recommended that the public wear cloth face masks to prevent the spread of COVID-19 in the form of source control [110]. The forced government COVID-19 prevention guidelines to the public have led both new and existing companies to manufacture reusable cloth face masks, some of which are impregnated or coated with nanoparticles including silver, copper, graphene, and zinc [111, 112]. The antiviral coatings market is valued at 0.5 billion dollars and is expected to grow to $1.3 billion by 2027, with silver coatings predicted to be the most profitable [113]. Some of the nanomaterial-coated and commercialized face masks are summarized in Table 1 [106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,123].
Schematic representation of the dissemination of SARS-CoV-2 between populations and the advancement in nanomaterials coatings for PPEs. a SARS-CoV-2 is potentially disseminated via viral aerosols generated from respiratory system of the infected host, which can travel in air longer than six feet in the air. b Strategies of advanced materials coating into facemasks can prevent infection of SARS-CoV-2. Various mechanisms are used to provide the facemask with self-sterilizing and self-cleaning capabilities. Reproduced with permission [105] under ACS COVID-19 subset
Mask wearing reduces the chance of entering other contaminants in the respiratory system. The viral load, which is filtered, totally depends on the type of mask used and its filtration efficiency. Since face masks and respirators should satisfy the performance criteria and recommended parameters specified by the American Society of Testing and Materials (ASTM) F2100 standard, in general, good-quality masks should possess the following five characteristics: (1) particulate filtration efficiency, (2) bacterial filtration efficiency, (3) fluid resistance, (4) differential pressure, and (5) flammability. These characteristics are dependent on the material used and the mask design. Different polymer fibres, such as polyester, polyethene, polyamide, polycarbonate, and polyphenylene oxide, manufacture masks [105]. Most conventional homemade masks use fabric materials such as cotton, silk, linen, tissue paper, and other household materials, such as towels and pillowcases. Unfortunately, these fabricated materials lack structural integrity and compromise particle filtration efficiency. Hence, extensive modification is required to ensure that these masks satisfy the demands during the crisis, including extended lifetime, reusability, and self-cleaning features to reduce unnecessary load on the environment.
The coating of nanomaterials on medical equipment is continuously accelerated in the past few years, and their applications as antiviral agents have significant public health and social importance. Organo-amine-functionalized silver nanoparticles have a soft edge for application in the coating of medical equipment such as antiviral and antimicrobial. Due to cationic, amine-functionalized silver nanoparticles attract the water nuclei simultaneously; silver nanoparticles bind with viruses and inactivate them. Further, functional organo-alkoxysilanes and polyethyleneimines in the presence of hydrophobic and hydrophilic moieties lead to a nanostructured network of organically modified silicates. Organo-amines such as 3-aminopropyltrimethoxysilane, 3 glycidoxypropyletrimethoxysilane, and polyethyleneimine-functionalized silver nanoparticles can be immobilized in preform siloxane polymer, which could be coated on face masks with immense stability and trap and kill efficiency. Acetone promotes the formation of siloxane polymer from 3-aminopropyltrimethoxysilane in chloroform and acetone solvents, but not in water. The siloxane polymer is soluble in acetone/chloroform; however, it may be easily converted into a thin film over suitable solid surfaces [124, 125]. 3-aminopropyltrimethoxysilane, and polyethyleneimine-functionalized silver nanoparticles can be immobilized in siloxane polymers in a précised and controlled nano-geometry self-assembled on any solid surface like cotton. This approach has been opted to coat the surgical mask with high filtration and antimicrobial efficiency [111]. Recently, Baselga et al. developed a mask by coating silver nanoparticles and taking PEI as an anchor molecule that binds the Ag-NPs and cotton fabric via electrostatic interaction (Fig. 5) [126]. The stability of the binding of the Ag-NPs to the fibres was corroborated using polypropylene, polyester viscose, and polypropylene-glass-spun-bonded mats as substrates, obtaining very low amounts of detached Ag-NPs in all cases. The antiviral coatings were tested against SARS-CoV-2, and obtained inactivation was 99.9% [126]. These face masks are reusable, durable, and have better filtration and antiviral efficiency. Nano-silver-coated face masks provide several opportunities for use in the current COVID-19 pandemic and future respiratory tract infecting viral disease prevention. During a medical crisis, medical personnel and staff could wear organo-amine-functionalized silver nanoparticle-coated cloth masks over N95 masks to preserve and protect the surface of the N95 for extended use when face shields are unavailable.
A Representing the Scanning Electron Microscopy of polypropylene spun-bonded fibres from a Ag-NPs–PEI-coated (using two different PEI concentrations and three Ag-NPs colloid concentrations) surgical mask. The micrographs of the samples obtained with a higher number of Ag-NPs and a higher PEI concentration show a significantly superior silver load. These images demonstrate that the concentration of Ag-NPs is as important as the amount of PEI to improve the silver incorporation yield. B Determination of the infective viral load (a) in materials coated with silver nanoparticles and the control after 2 h and (b) is coated spunbond after 10 min, after 2 h after infection, and after 4 h after infection with the SARS-CoV-2 virus at room temperature. Adapted with permission from [126] under a common creative attribution licence
Concluding Remark
This review summarizes factors influencing the viral particle adsorption on fomites, previously reported antiviral activity and the possibility of amine-functionalized silver nanoparticle coating materials for face masks that inactivate viruses. In addition, the strategies involved in developing antiviral coatings, like modifying the surface of a substrate via the incorporation of functional silver nanoparticles, were discussed. A significant problem that needs considerable attention is the virus particles' long-term persistence on the face masks' surface layer, putting them at a higher risk level during their usage and disposal. Hence, manufacturing optimized face masks by applying metal ions, consisting of nanoparticles on the surface of the filtering layer, could be considered a viable approach for the immediate elimination of viruses. Moreover, self-cleaning coatings on the filtering layer of the masks could be applied to avoid the attachment of infectious microdroplets on face masks. The SARS-CoV-2 responsible for the current pandemic is transmitted through the air via microdroplets exhaled by infected persons, and proximal surfaces can spread the virus from one person to another. Furthermore, several reports indicated that the virus remains viable on various surfaces for extended periods, for days and even longer. Therefore, there is a clear need for durable antiviral coatings that can be sprayed or painted on surfaces, like paint or varnish, preventing viral transmission and the role of silver nanoparticles-siloxane polymer derived from amine-functionalized trialkoxysilane in volatile solvents are of great significance to meet these requirements.
Abbreviations
- PPEs:
-
Personal protective equipment
- BPEI:
-
Branched polyethyleneimines
- LPEI:
-
Linear polyethyleneimines
- WHO:
-
World Health Organisation
- Ag-NPs:
-
Silver nanoparticles
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- GO:
-
Graphene oxide
- 3-APTMS:
-
3-Aminopropyltrimethoxysilane
- 3-GPTMS:
-
3-Glycidoxypropyltrimethoxysilane
- VPP:
-
1-Vinyl 2 pyrrolidone
- CS:
-
Chitosan
References
WHO report-98, Johns Hopkins University, www.coronavirus.jhu.edu/map.html January, 2022.
WHO COVID-19 update dashboard, https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed Jan 2022.
W. Muhammad, M.A. Khan, M. Nazir, A. Siddiquah, S. Mushtaq, S.S. Hashmi, B.H. Abbasi, Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: in-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mater. Sci. Eng. C Mater. Biol. Appl. 103, 109740 (2019). https://doi.org/10.1016/j.msec.2019.109740
J. Shi, A.R. Votruba, O.C. Farokhzad, R. Langer, Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 10, 3223–3230 (2010). https://doi.org/10.1021/nl102184c
W. Muhammad, N. Ullah, M. Haroon, B.H. Abbasi, Optical, morphological and biological analysis of zinc oxide nanoparticles (ZnO NPs) using Papaver somniferum L. RSC Adv. 9, 29541–29548 (2019). https://doi.org/10.1039/c9ra04424h
W. Muhammad, M. Khan, N. Ullah, The significance of phyto-fabricated nanoparticles in curing gastric ulcer. Nanoscale Rep. 2, 1–2 (2019). https://doi.org/10.26524/nr1911
H.Y. Huang, C.H. Fan, M. Li, H.L. Nie, F.B. Wang, H. Wang, R. Wang, J.B. Xia, Zheng et al., COVID-19: a call for physical scientists and engineers. ACS Nano 14, 3747 (2020). https://doi.org/10.1021/acsnano.0c02618
R. Pemmada, X. Zhu, M. Dash, Y. Zhou, S. Ramakrishna, X. Peng, V. Thomas, S. Jain, H.S. Nanda, Science-based strategies of antiviral coatings with viricidal properties for the COVID-19 like pandemics. Materials 13, 4041 (2021). https://doi.org/10.3390/ma13184041
S. Galdiero, A. Falanga, M. Vitiello, M. Cantisani, V. Marra, M. Galdiero, Silver nanoparticles as potential antiviral agents. Molecules 16, 8894–8918 (2011). https://doi.org/10.3390/molecules16108894
T. Kuiken, E.P. Vejerano, S.P. McGinnis, M.F. Hochella Jr., D. Rejeski, M.S. Hull, Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 6, 1769–1780 (2015). https://doi.org/10.3762/bjnano.6.181
Q.L. Feng, J. Wu, G.Q. Chen, F.Z. Cui, T.N. Kim, J.O. Kim, A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 52, 662–668 (2000)
J.R. Morones, J.L. Elechiguerra, A. Camacho, K. Holt, J.B. Kouri, J.T. Ramírez, M.J. Yacaman, The bactericidal effect of silver nanoparticles. Nanotechnology 16, 2346–2353 (2005). https://doi.org/10.1088/0957-4484/16/10/059
I. Sondi, B. Salopek-Sondi, Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275, 177–182 (2004). https://doi.org/10.1016/j.jcis.2004.02.012
D. Wei, W. Sun, W. Qian, Y. Ye, X. Ma, The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 344, 2375–2382 (2009). https://doi.org/10.1016/j.carres.2009.09.001
A. Panacek, L. Kvítek, R. Prucek, M. Kolar, R. Vecerova, N. Pizúrova, V.K. Sharma, T. Nevecna, R. Zboril, Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 110, 16248–16253 (2006). https://doi.org/10.1021/jp063826h
S. Pal, Y.K. Tak, J.M. Song, Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73, 1712–1720 (2007). https://doi.org/10.1128/AEM.02218-06
K.Y. Yoon, J. HoonByeon, J.H. Park, J. Hwang, Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 373, 572–575 (2007). https://doi.org/10.1016/j.scitotenv.2006.11.007
A.K. Tiwari, M.K. Gupta, G. Pandey, R. Tilak, R.J. Narayan, P.C. Pandey, Size and zeta potential clicked germination attenuation and anti-sporangiospores activity of PEI-functionalized silver nanoparticles against COVID-19 associated Mucorales (Rhizopus arrhizus). Nanomaterials 12, 2235 (2022). https://doi.org/10.3390/nano12132235
A.R. Shahverdi, A. Fakhimi, H.R. Shahverdi, S. Minaian, Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 3, 168–171 (2007). https://doi.org/10.1016/j.nano.2007.02.001
M. Banoee, S. Seif, Z.E. Nazari, P. Jafari-Fesharaki, H.R. Shahverdi, A. Moballegh, K.M. Moghaddam, A.R. Shahverdi, ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J. Biomed. Mater. Res. B Appl. Biomater. 93, 557–561 (2010). https://doi.org/10.1002/jbm.b.31615
A.M. Fayaz, K. Balaji, M. Girilal, R. Yadav, P.T. Kalaichelvan, R. Venketesan, Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine 6, 103–109 (2010). https://doi.org/10.1016/j.nano.2009.04.006
W.J. Plieth, Electrochemical properties of small clusters of metal atoms and their role in the surface enhanced Raman scattering. J. Phys. Chem. 86, 3166–3170 (1982)
K.D. Kim, D.N. Han, H.T. Kim, Optimization of experimental conditions based on the Taguchi robust design for the formation of nano-sized silver particles by chemical reduction method. Chem. Eng. J. 104, 55–61 (2004)
A.M. El Badawy, K.G. Scheckel, M. Suidan, T. Tolaymat, The impact of stabilization mechanism on the aggregation kinetics of silver nanoparticles. Sci. Total Environ. 429, 325–331 (2012). https://doi.org/10.1016/j.scitotenv.2012.03.041
D.A. Brian, R.S. Baric, Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 287, 1–30 (2005). https://doi.org/10.1007/3-540-26765-4_1
N.J. Hardenbrook, P. Zhang, A structural view of the SARS-CoV-2 virus and its assembly. Curr. Opin. Virol. 52, 123–134 (2022). https://doi.org/10.1016/j.coviro.2021.11.011
A. Kambhampati, M. Koopmans, B.A. Lopman, Burden of norovirus in healthcare facilities and strategies for outbreak control. J. Hosp. Infect. 89, 296–301 (2015). https://doi.org/10.1016/j.jhin.2015.01.011
P. Vasickova, I. Pavlik, M. Verani, A. Carducci, Issues concerning survival of viruses on surfaces. Food Environ. Virol. 2, 24–34 (2010). https://doi.org/10.1007/s12560-010-9025-6
L.F. Kiseleva, Survival of poliomyelitis, ECHO and Coxsackie viruses in some food products. Vopr Pitan 30, 58–61 (1971)
S. Firquet et al., Survival of enveloped and non-enveloped viruses on inanimate surfaces. Microbes Environ. 30, 140–144 (2015). https://doi.org/10.1264/jsme2.ME14145
P.D. Rakowska, M. Tiddia, N. Faruqui et al., Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2, 53 (2021). https://doi.org/10.1038/s43246-021-00153-y
J. Sizun, M.W.N. Yu, P.J. Talbot, Survival of human coronaviruses 229E and OC43 in suspension and after drying on surfaces: a possible source of hospital-acquired infections. J. Hosp. Infect. 46, 55–60 (2000). https://doi.org/10.1053/jhin.2000.0795
J. Zhuang, Y. Jin, Virus retention and transport as influenced by different forms of soil organic matter. J. Environ. Quality 32, 816–823 (2003). https://doi.org/10.2134/jeq2003.8160
M.H. Wolff, S.A. Sattar, O. Adegbunrin, J. Tetro, Coronaviruses with Special Emphasis on First Insights Concerning SARS (Springer, Berlin, 2005), pp.201–212
A. Tiwari, D.P. Patnayak, Y. Chander, M. Parsad, S.M. Goyal, Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 50, 284–287 (2006). https://doi.org/10.1637/7453-101205R.1
B. Bean et al., Survival of influenza viruses on environmental surfaces. J. Infect. Dis. 146, 47–51 (1982). https://doi.org/10.1093/infdis/146.1.47
F.X. Abad, R.M. Pintó, A. Bosch, Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 60, 3704–3710 (1994). https://doi.org/10.1128/aem.60.10.3704-3710.1994
S.M. Duan et al., Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 16, 246–255 (2003)
T.P. Weber, N.I. Stilianakis, Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J. Infect. 57, 361–373 (2008). https://doi.org/10.1016/j.jinf.2008.08.013
A. Chin et al., Stability of SARS-CoV-2 in different environmental conditions. Lancet 1, E10 (2020). https://doi.org/10.1016/S2666-5247(20)30003-3
S.Y. Ren et al., Stability and infectivity of coronaviruses in inanimate environments. World J. Clin. Cases 8, 1391–1399 (2020). https://doi.org/10.12998/wjcc.v8.i8.1391
X. Xue, J.K. Ball, C. Alexander, M.R. Alexander, All surfaces are not equal in contact transmission of SARS-CoV-2. Matter 3, 1433–1441 (2020). https://doi.org/10.1016/j.matt.2020.10.006
M.Y.Y. Lai, P.K.C. Cheng, W.W.L. Lim, Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 41, e67–e71 (2005). https://doi.org/10.1086/433186
E. Joonaki, A. Hassanpouryouzband, C.L. Heldt, O. Areo, Surface chemistry can unlock drivers of surface stability of SARS-CoV-2 in a variety of environmental conditions. Chem 6, 2135–2146 (2020). https://doi.org/10.1016/j.chempr.2020.08.001
C.L. Heldt, A. Zahid, K.S. Vijayaragavan, X. Mi, Experimental and computational surface hydrophobicity analysis of a non-enveloped virus and proteins. Colloids Surf. B Biointerfaces 153, 77–84 (2017). https://doi.org/10.1016/j.colsurfb.2017.02.011
H. Shi, V.V. Tarabara, Charge, size distribution and hydrophobicity of viruses: effect of propagation and purification methods. J. Virol. Methods 256, 123–132 (2018). https://doi.org/10.1016/j.jviromet.2018.02.008
D. Chattopadhyay, S. Chattopadhyay, W.G. Lyon, J.T. Wilson, Effect of surfactants on the survival and sorption of viruses. Environ. Sci. Technol. 36, 4017–4024 (2002). https://doi.org/10.1021/es0114097
M. Rai, A. Yadav, A. Gade, Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27, 76–83 (2009). https://doi.org/10.1016/j.biotechadv.2008.09.002
M.K. Rai, S.D. Deshmukh, A.P. Ingle, A.K. Gade, Silver nanoparticles, the powerful nano weapon against multidrug-resistant bacteria. J. Appl. Microbiol. 112, 841–852 (2012). https://doi.org/10.1111/j.1365-2672.2012.05253.x
S. Galdiero, A. Falanga, M. Vitiello et al., Silver nanoparticles as potential antiviral agents molecules. Molecules 16, 8894–8918 (2011). https://doi.org/10.3390/molecules16108894
N.G. Portney, M. Ozkan, Nano-oncology, drug delivery, imaging, and sensing. Anal Bioanal. Chem. 384, 620–630 (2006). https://doi.org/10.1007/s00216-005-0247-7
S. Gaikwad, A. Ingle, A. Gade et al., Antiviral activity of myco synthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 8, 4303–4314 (2013). https://doi.org/10.2147/IJN.S50070
L. Lu, R.W. Sun, R. Chen et al., Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 13, 253–262 (2008)
J.L. Elechiguerra, J.L. Burt, J.R. Morones et al., Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 3, 6 (2005). https://doi.org/10.1186/1477-3155-3-6
H.H. Lara, N.V. Ayala-Nunez, L. Ixtepan-Turrent, C. Rodriguez-Padilla, Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 8, 1 (2010). https://doi.org/10.1186/1477-3155-8-1
R.W. Sun, R. Chen, N.P. Chung et al., Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. 40, 5059–5061 (2005). https://doi.org/10.1039/b510984a
D. Baram-Pinto, S. Shukla, N. Perkas et al., Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug. Chem. 20, 1497–1502 (2009). https://doi.org/10.1021/bc900215b
L. Sun, A.K. Singh, K. Vig et al., Silver nanoparticles inhibit replication of respiratory sincitial virus. J. Biomed. Biotechnol. 4, 149–158 (2008). https://doi.org/10.1166/jbn.2008.012
J.L. Speshock, R.C. Murdock, L.K. Braydich-Stolle et al., Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 8, 19 (2010). https://doi.org/10.1186/1477-3155-8-19
J.V. Rogers, C.V. Parkinson, Y.W. Choi et al., A preliminary assessment of silver nanoparticles inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 3, 129–133 (2008). https://doi.org/10.1007/s11671-008-9128-2
P. Mehrbod, N. Motamed, M. Tabatabaian et al., In vitro antiviral effect of ‘“Nanosilver”’ on influenza virus. Daru 17, 88–93 (2009)
D.X. Xiang, Q. Chen, L. Pang, C.L. Zheng, Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Virol. Methods 178, 137–142 (2011). https://doi.org/10.1016/j.jviromet.2011.09.003
T.R. Sinclair, S.K. van den Hengel, B.G. Raza, S.A. Rutjes, A.M. de Roda Husman, W.J.G.M. Peijnenburg, H.E.D.W. Roesink, W.M. de Vos, Surface chemistry-dependent antiviral activity of silver nanoparticles. Nanotechnology (2021). https://doi.org/10.1088/1361-6528/ac03d6
D. Morris, M. Ansar, J. Speshock, T. Ivanciuc, Y. Qu, A. Casola, R. Garofalo, Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses 11, 732 (2019). https://doi.org/10.3390/v11080732
E. Husanu, C. Chiappe, A. Bernardini, V. Cappello, M. Gemmi, Synthesis of colloidal Ag nanoparticles with citrate based ionic liquids as reducing and capping agents. Colloids Surf. A 538, 506–512 (2018). https://doi.org/10.1016/j.colsurfa.2017.11.033M
I. Tabujew, K. Peneva, CHAPTER 1: Functionalization of Cationic Polymers for Drug Delivery Applications, in Cationic Polymers in Regenerative Medicine (2014), pp. 1–29. https://doi.org/10.1039/9781782620105-00001
P.C. Pandey, Indian Patent, A process for in-situ generation of noble metal nanoparticles and thereafter core shell ofthe same. Indian Patent 331496, granted on 05/08/2020 and filed on 04/10/2010
P.C. Pandey, D.S. Chauhan, 3-Glycidoxypropyltrimethoxysilane mediated in situ synthesis of noble metal nanoparticles: application to hydrogen peroxide sensing. Analyst 137, 376–385 (2012)
P.C. Pandey, A.K. Tiwari, M.K. Gupta, G. Pandey, R.J. Narayan, Effect of the organic functionality on the synthesis and antimicrobial activity of silver nanoparticles. Nano LIFE. 10, 2050002 (2020)
L.J. Luo, T.Y. Lin, C.H. Yao, P.Y. Kuo, M. Matsusaki, S.G. Harroun, C.C. Huang, J.Y. Lai, Dual-functional gelatin-capped silver nanoparticles for antibacterial and antiangiogenic treatment of bacterial keratitis. J. Colloid Interface Sci. 536, 112–126 (2019). https://doi.org/10.1016/j.jcis.2018.10.041
B. Brissault, A. Kichler, C. Guis, C. Leborgne, O. Danos, H. Cheradame, Synthesis of linear polyethylenimine derivatives for DNA transfection. Bioconjug. Chem. 14, 581–587 (2003). https://doi.org/10.1021/bc0200529
L. Tauhardt, K. Kempe, K. Knop, E. Altuntas, M. Jaeger, S. Schubert, D. Fischer, U.S. Schubert, Linear polyethyleneimine: optimized synthesis and characterization—on the way to “Pharmagrade” batches. Macromol. Chem. Phys. 212, 1918–1924 (2011)
G.D. Jones, A. Langsjoen, M.M.C. Neumann, J. Zomlefer, The polymerization of ethyleneimine. J. Org. Chem. 9, 125–147 (1944)
A.K. Tiwari, M.K. Gupta, G. Pandey, R.J. Narayan, P.C. Pandey, Molecular weight of polyethyleneimine-dependent transfusion and selective antimicrobial activity of functional silver nanoparticles. J. Mater. Res. 35, 2405–2415 (2020). https://doi.org/10.1557/jmr.2020.183
P.C. Pandey, G. Pandey, R.J. Narayan, Controlled synthesis of polyethylenimine coated gold nanoparticles: application in glutathione sensing and nucleotide delivery. J. Biomed. Mater. Res. B Appl. Biomater. 105, 1191–1199 (2017). https://doi.org/10.1002/jbm.b.33647
Z. Liu, Y. Wang, Y. Zu, Y. Fu, N. Li, N. Guo, R. Liu, Y. Zhang, Synthesis of polyethylenimine (PEI) functionalized silver nanoparticles by a hydrothermal method and their antibacterial activity study. Mater. Sci. Eng. C Mater. Biol. Appl. 42, 31–37 (2014). https://doi.org/10.1016/j.msec.2014.05.007
K. Kim, H.B. Lee, J.W. Lee, K.S. Shin, Poly(ethylenimine)-stabilized silver nanoparticles assembled into 2-dimensional arrays at water-toluene interface. J. Colloid Interface Sci. 345, 103–108 (2010). https://doi.org/10.1016/j.jcis.2010.01.039
S. Akbari-Alavijeh, R. Shaddel, S.M. Jafari, Encapsulation of food bioactive and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocolloids 105, 105774 (2020). https://doi.org/10.1016/j.foodhyd.2020.105774
M. Hosseinnejad, S.M. Jafari, Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 85, 467–475 (2016). https://doi.org/10.1016/j.ijbiomac.2016.01.022
H. Joz Majidi, A. Babaei, Z. Arab Bafrani, D. Shahrampour, E. Zabihi, S.M. Jafari, Investigating the best strategy to diminish the toxicity and enhance the antibacterial activity of graphene oxide by chitosan addition. Carbohydr. Polym. 225, 115220 (2019). https://doi.org/10.1016/j.carbpol.2019.115220
J. Wongpreecha, D. Polpanich, T. Suteewong, C. Kaewsaneha, P. Tangboriboonrat, One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 99, 641–648 (2018). https://doi.org/10.1016/j.carbpol.2018.07.039
S.U. Islam, B.S. Butola, D. Verma, Facile synthesis of chitosan-silver nanoparticles onto linen for antibacterial activity and free-radical scavenging textiles. Int. J. Biol. Macromol. 133, 1134–1141 (2019). https://doi.org/10.1016/j.ijbiomac.2019.04.186
S. Szunerits, A. Barras, M. Khanal, Q. Pagneux, R. Boukherroub, Nanostructures for the inhibition of viral infections. Molecules 20, 14051–14081 (2015). https://doi.org/10.3390/molecules200814051
L. Lu, R.W. Sun, R. Chen, C.K. Hui, C.M. Ho, J.M. Luk, G.K. Lau, C.M. Che, Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 13, 253–262 (2008)
S. Rafiei, S.E. Rezatofighi, M. Roayaei Ardakani, S. Rastegarzadeh, Gold nanoparticles impair foot-and-mouth disease virus replication. IEEE Trans. Nanobiosci. 15, 34–40 (2016). https://doi.org/10.1109/TNB.2015.2508718
A.S. Levina, M.N. Repkova, E.V. Bessudnova, E.I. Filippova, N.A. Mazurkova, V.F. Zarytova, High antiviral effect of TiO2·PL-DNA nanocomposites targeted to conservative regions of (-) RNA and (+) RNA of influenza A virus in cell culture. Beilstein J. Nanotechnol. 7, 1166–1173 (2016). https://doi.org/10.3762/bjnano.7.108
D. Botequim, J. Maia, M.M. Lino, L.M. Lopes, P.N. Simões, L.M. Ilharco, L. Ferreira, Nanoparticles and surfaces presenting antifungal, antibacterial and antiviral properties. Langmuir 28, 7646–7656 (2012). https://doi.org/10.1021/la300948n
V. Lysenko, V. Lozovski, M. Lokshyn, Y.V. Gomeniuk, A. Dorovskih, N. Rusinchuk, Y. Pankivska, O. Povnitsa, S. Zagorodnya, V. Tertykh et al., Nanoparticles as antiviral agents against adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 9, 025021 (2018)
T.H. Sucipto, S. Churrotin, H. Setyawati, T. Kotaki, F. Martak, S. Soegijanto, In vitro study: effect of Cobalt (II) chloride against dengue virus type 1 in vero cells. Indones. J. Trop. Infect. Dis. 6, 84 (2017)
N.A. Mazurkova, Y.E. Spitsyna, N.V. Shikina, Z.R. Ismagilov, S.N. Zagrebel’nyi, E.I. Ryabchikova, Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol. Russ. 5, 417 (2010)
R.L. Hu, S.R. Li, F.J. Kong, R.J. Hou, X.L. Guan, F. Guo, Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet. Mol. Res. 13, 7022–7028 (2014). https://doi.org/10.4238/2014.March.19.2
P. Kuppusamy, S.J. Ichwan, N.R. Parine, M.M. Yusoff, G.P. Maniam, N. Govindan, Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. J. Environ. Sci. 29, 151–157 (2015). https://doi.org/10.1016/j.jes.2014.06.050
A. Akbarzadeh, L. Kafshdooz, Z. Razban, A. Dastranj Tbrizi, S. Rasoulpour, R. Khalilov, T. Kavetskyy, S. Saghfi, A.N. Nasibova, S. Kaamyabi, T. Kafshdooz, An overview application of silver nanoparticles in inhibition of herpes simplex virus. Artif. Cells Nanomed. Biotechnol. 46, 263–267 (2018). https://doi.org/10.1080/21691401.2017.1307208
N. Duran, M. Duran, C.E. de Souza, Silver and silver chloride nanoparticles and their anti-tick activity: a mini review. J. Braz. Chem. Soc. 28, 927–932 (2017)
C.B. Jackson, M. Farzan, B. Chen, H. Choe, Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell. Biol. 23(1), 3–20 (2022). https://doi.org/10.1038/s41580-021-00418-x
G. Chauhan, M.J. Madou, S. Kalra, V. Chopra, D. Ghosh, S.O. Martinez-Chapa, Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano 14, 7760–7782 (2020). https://doi.org/10.1021/acsnano.0c04006
L. Chen, J. Liang, An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C Mater. Biol. Appl. 112, 110924 (2020). https://doi.org/10.1016/j.msec.2020.110924
S. Gurunathan, M. Qasim, Y. Choi, J.T. Do, C. Park, K. Hong, J.H. Kim, H. Song, Antiviral potential of nanoparticles-can nanoparticles fight against coronaviruses? Nanomaterials 10, 1645 (2020). https://doi.org/10.3390/nano10091645
V. Cagno, P. Andreozzi, M. D’Alicarnasso, P. Jacob Silva, M. Mueller, M. Galloux, R. Le Goffic, S.T. Jones, M. Vallino, J. Hodek, J. Weber, S. Sen, E.R. Janeček, A. Bekdemir, B. Sanavio, C. Martinelli, M. Donalisio, M.A. RameixWelti, J.F. Eleouet, Y. Han, L. Kaiser, L. Vukovic, C. Tapparel, P. Král, S. Krol, D. Lembo, F. Stellacci, Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 17, 195–203 (2018). https://doi.org/10.1038/nmat5053
A.K. Tiwari, A. Mishra, G. Pandey, M.K. Gupta, P.C. Pandey, Nanotechnology: a potential weapon to fight against COVID-19. Part Part Syst. Charact. 39, 2100159 (2022). https://doi.org/10.1002/ppsc.202100159
WHO Transmission of SARS-CoV-2: Implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sarscov-2-implications-for-infection-prevention-precautions
Y. Liu, Z. Ning, Y. Chen, M. Guo, Y. Liu, N.K. Gali, L. Sun, Y. Duan, J. Cai, D. Westerdahl, X. Liu, K. Xu, K.F. Ho, H. Kan, Q. Fu, K. Lan, Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 582, 557–560 (2020). https://doi.org/10.1038/s41586-020-2271-3
N. van Doremalen, T. Bushmaker, D.H. Morris, M.G. Holbrook, A. Gamble, B.N. Williamson, A. Tamin, J.L. Harcourt, N.J. Thornburg, S.I. Gerber, J.O. Lloyd-Smith, E. de Wit, V.J. Munster, Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382, 1564–1567 (2020). https://doi.org/10.1056/NEJMc2004973
A. Konda, A. Prakash, G.A. Moss, M. Schmoldt, G.D. Grant, S. Guha, Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano 14, 6339–6347 (2020). https://doi.org/10.1021/acsnano.0c03252
M. Karmacharya, S. Kumar, O. Gulenko, Y.K. Cho, Advances in facemasks during the COVID-19 pandemic era. ACS Appl. Bio Mater. 4, 3891–3908 (2021). https://doi.org/10.1021/acsabm.0c01329
J. Lu, J. Gu, K. Li, C. Xu, W. Su, Z. Lai, D. Zhou, C. Yu, B. Xu, Z. Yang, COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 26, 1628–1631 (2020). https://doi.org/10.3201/eid2607.200764
B. Stephens, J.A. Siegel, Ultrafine particle removal by residential heating, ventilating, and air-conditioning filters. Indoor Air 23(6), 488–497 (2013). https://doi.org/10.1111/ina.12045
D.K. Chu, E.A. Akl, S. Duda et al., COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 395(10242), 1973–1987 (2020). https://doi.org/10.1016/S0140-6736(20)31142-9
Face Mask Market Size, Share, Trends | Industry Report, 2021−2027. https://www.alliedmarketresearch.com/face-mask-marketA06289. Accessed Feb 2022
About Cloth Face Coverings | CDC. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/about-face-coverings.html. Accessed Feb 2022
M.H. Chua, W. Cheng, S.S. Goh, J. Kong, B. Li, J.Y.C. Lim, L. Mao, S. Wang, K. Xue, L. Yang, E. Ye et al., Face masks in the new COVID-19 normal: materials, testing, and perspectives. Research (2020). https://doi.org/10.34133/2020/7286735
Face masks made in USA help small business - The Washington Post. https://www.washingtonpost.com/business/2020/08/05/facemasks-made-usa/. Accessed Feb 2022
Anti-Viral Coatings Market Size | Industry Growth & Forecast, 2027. https://www.alliedmarketresearch.com/anti-viral-coatingsmarket-A06722. Accessed Feb 2022
M. Swindell, NOTICE OF PESTICIDE: X Registration • Reregistration SilvaDurTM 930 Antimicrobial Name and Address of Registrant (Include ZIP Code). Accessed Feb 2022
https://proextintor.es/wpcontent/uploads/2020/10/SILVADUR-930-FLEX_TDS-2.pdf
Nightingale - Masks designed for Next-Level Comfort and Protection. https://www.nightingalesafe.com/. Accessed Feb 2022
A.L. Chapman, OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION; 2016.
Jaanuu Antimicrobial-Finished Face Masks: Buy 1 Donate 1. For Adults and Children. https://www.jaanuu.com/facemasks?color=graphite&size=adult. Accessed Feb 2022
La Sportiva Stratos Mask. https://www.sportiva.com/stratosmask.html. Accessed Feb. 2022
Viral Guard Pro. https://viralguardpro.com/. Accessed Feb 2022
HeiQ V-Block + Multi Hi-Tech Washable Mask, duopack (USA) − HeiQ Viroblock Mask. https://shop.heiq-viroblock-masks.com/collections/masks-available-in-usa/products/heiqviroblockwashable-mask-rw-dwr. Accessed Feb 2022
The ISKO VitalTM Supreme Face Cover | ISKOVital.com. https://iskovital.com/collections/face-covers/products/supremeface-cover. Accessed Feb 2022
Silverlon Face Mask. https://www.thresholdmedical.com/product/silverlon-face-mask/1. Accessed Feb 2022
A.K. Tiwari, M.K. Gupta, G. Pandey, P.C. Pandey, Siloxane-silver nanofluid as potential self-assembling disinfectant: a preliminary study on the role of functional alkoxysilanes. Nanoarchitectonics 4(1), 1–15 (2022). https://doi.org/10.37256/nat.4120231576
P.C. Pandey, Antimicrobial and antiviral mask, https://www.youtube.com/watch?v=ViQ9ivQ8msg. Accessed Apr 2020
M. Baselga, I. Uranga-Murillo, D. de Miguel, M. Arias, V. Sebastián, J. Pardo, M. Arruebo, Silver nanoparticles—polyethyleneimine-based coatings with antiviral activity against SARS-CoV-2: a new method to functionalize filtration media. Materials 15, 4742 (2022). https://doi.org/10.3390/ma15144742
Funding
No funding available for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tiwari, A.K., Gupta, M.K., Pandey, G. et al. Amine-Functionalized Silver Nanoparticles: A Potential Antiviral-Coating Material with Trap and Kill Efficiency to Combat Viral Dissemination (COVID-19). Biomedical Materials & Devices 1, 618–632 (2023). https://doi.org/10.1007/s44174-022-00044-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-022-00044-x