Abstract
All over the world, from America to the Mediterranean Sea, the plant pathogen Xylella fastidiosa represents one of the most difficult challenges with many implications at ecological, agricultural, and economic levels. X. fastidiosa is a rod-shaped Gram-negative bacterium belonging to the family of Xanthomonadaceae. It grows at very low rates and infects a wide range of plants thanks to different vectors. Insects, through their stylets, suck a sap rich in nutrients and inject bacteria into xylem vessels. Since, until now, no antimicrobial treatment has been successfully applied to kill X. fastidiosa and/or prevent its diffusion, in this study, antimicrobial blue light (aBL) was explored as a potential anti-Xylella tool. Xylella fastidiosa subsp. pauca Salento-1, chosen as a model strain, showed a certain degree of sensitivity to light at 410 nm. The killing effect was light dose dependent and bacterial concentration dependent. These preliminary results support the potential of blue light in decontamination of agricultural equipment and/or plant surface; however, further investigations are needed for in vivo applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Xylella fastidiosa is one of the most harmful alien pathogens originating from the American continent affecting grapevine, citrus, coffee, and olive trees [1]. It is transmitted by insects belonging to families of Cicadellidae, Cicadellinae, and Cercopoidae that assimilate amino acids, organic acids, and sugars from sucking xylem sap [2]. The bacterium is injected into the xylem vessels of leaf petioles after the penetration of the insect vector’s stylet [3]. X. fastidiosa is a Gram-negative rod-shaped bacterium belonging to the Xanthomonas family. It is a slow-growing and fastidious bacterium that colonizes the xylem of its host plants moving against water flow, forms biofilms, and occludes the vessels, killing the plants [4].
In 2013, X. fastidiosa was detected the first time in Europe affecting olive orchards of the Salento Peninsula in the region of Apulia, causing olive quick decline syndrome (OQDS) [5]. Although complex abiotic and biotic factors were strongly related to this syndrome, the fulfillment of Koch’s postulates found X. fastidiosa as the etiological agent of OQDS [6]. Among the hosts of X. fastidiosa, many endemic Mediterranean species in addition to oil tree have been found: both ornamental and crop species such as cherry (Prunus avium), oleander (Nerium oleander), and almond (Prunus dulcis) [5]. In 2015, X. fastidiosa has been detected in the island of Corsica and in continental France [7], and in 2016 in the Balearic Islands in Spain with a big delay compared to its hypothesized introduction 23 years before [8]. Further outbreaks in Israel, Tuscany, and Portugal have been more recently reported [8]. On the basis of 16S-23S rRNA internal transcribed spacer (ITS) sequencing, different subspecies have been proposed and among these the most harmful are fastidiosa, pauca, multiplex, and sandyi [9].
Nowadays, the European Community is facing the consequences of such enemy that are devastating from ecological, agricultural, and economic point of view. Until now, no cure has been found for this pest, but different strategies have been proposed and authorized. In particular, the decision (EU) 2015/789 required the elimination of plants in infected areas, within 100 m radius around X. fastidiosa-positive plant, regardless of their phytosanitary status. In addition, the vector control can be achieved through the use of insecticides for the treatment of olive trees, such as Kaolin 95% WP p/p, Deltamethrin 10% (EC) p/v, and Lambda-Cyhalothrin 10% (CS) p/v [8]. Additional controls are based on propagation of plant material pathogen-free or resistant plant cultivars, territory demarcation, and bactericidal treatments [10].
Although, in literature, several studies report antimicrobial treatments aimed at killing X. fastidiosa, new efforts are undoubtedly needed to manage this enemy. Furthermore, there is evidence of development of resistance to copper-based antimicrobial compounds often used for crop protection, especially in Xanthomonas family. The discovery of novel bactericidal compounds or treatments is essential to counteract the spread of resistant strains. Promising results have been obtained by da Silva et al. that injected in vivo, using a syringe, ruthenium and magnesium complexes that drastically reduced the detection, through real-time quantitative PCR, of X. fastidiosa [11]. The same culture-independent method was exploited by Barò et al. to verify the anti-Xylella potential of peptide BP100 and fragments of cecropin, magainin, or melittin that showed to be good candidates. Indeed, antimicrobial peptides from plants represent promising compounds with low cytotoxicity and low probability to induce resistance mechanisms in microbial targets [12]. The screening of synthetic peptides from libraries could represent a good reservoir of putative antimicrobials [13]. The blocking of the afimbrial adhesin XadA2 of X. fastidiosa successfully inhibited biofilm formation. The combination of chitosan nano-materials combined with fosetyl-Al crystals showed antibiofilm activity in X. fastidiosa subsp. pauca and subsp. fastidiosa [14].
In this context, the antimicrobial blue light (aBL) could represent a novel strategy to be considered. This approach exploits the ability of visible light alone, particularly in the range of blue light from 400 to 500 nm, in damaging microbial pathogens, and leading to cell death. Many microbial species demonstrated to be sensitive to blue light treatment, especially those causing human infections such as ESKAPE pathogens including P. aeruginosa, S. aureus, K. pneumoniae, and A. baumannii [15]. It has been hypothesized that aBL mechanism of action is based on the generation of photooxidative stress upon the excitation of endogenous molecules that behave as photosensitizing agents [16]. In different bacterial and fungal species, putative endogenous molecules acting as photosensitizers (PSs) have been recognized, belonging to chemical classes of porphyrins and/or flavins. Indeed, the main types of endogenous compounds found in bacteria and yeasts are protoporphyrin IX, coproporphyrin I, and coproporphyrin III [15, 17]. In the last years, a wider literature reports the antimicrobial power of blue light in inhibiting the growth of bacteria, with many potential applications in agri-food field, livestock, and industrial sectors, for the treatment of plant and animal pathogens, and for disinfection purposes [15, 18]. Many studies suggested that no evidence of microbial resistance toward aBL was found in P. aeruginosa, A. baumannii, and C. albicans after sublethal doses of blue light [19,20,21]. However, a certain degree of tolerance to aBL was found in S. aureus. [22].
The authors previously showed that light at 410 nm was efficient in inhibiting the growth of an opportunistic pathogen such as Pseudomonas aeruginosa [23]. In this study, the sensitivity of X. fastidiosa to light at 410 nm was investigated.
2 Materials and methods
2.1 Bacterial strains and culture conditions
Xylella fastidiosa subsp. pauca Salento-1 isolate (NCCPB No. 4595 LMG 29352) [24, 25] was grown in Buffered Charcoal Yeast Extract (BCYE) Agar medium (LaBM) for 15–20 days at 28 °C. For long-term storage, glycerol stocks of X. fastidiosa cells were prepared in 1X PBS buffer (KH2PO4/K2HPO4 10 mM, NaCl 137 mM, KCl 2.7 mM, pH 7.4) with a final glycerol concentration of 15% (v/v) and stored at − 80 °C.
2.2 Light sources
The lighting unit device used to photo-inactivate bacteria is equipped with a head composed by 25 high-power LEDs with maximum emission peak at 410 nm and allows the uniform irradiation of a square area of 75 mm × 75 mm (Fig. 1A, and C, black line). The source was placed 10 cm above the Petri dishes to be treated. At this distance, the irradiance was 100 mW/cm2, measured with the spectral light meter MSC15 (Gigahertz Optik GmbH). A 50 W incandescent lamp emitting white light (T = 2900 K) with negligible emission in the blue region was used for irradiation control experiments to assess the sensitivity of X fastidiosa to wavelengths other than that under investigation. The source was placed 8 cm above the Petri dishes (Fig. 1B) to obtain the same heating effect as the blue light (see later). Also in this case, the geometry of the system allows uniform illumination of an area larger than the Petri dishes used for the experiments. The relevant emission spectrum is shown in Fig. 1C (red solid line) for the interval 350–830 nm where the MSC15 spectral light meter is sensitive, with a measured irradiance of 11 mW/cm2. Figure 1C (red dash-dotted line) shows the calculated emission spectrum of a black body with a temperature of 2900 K, rescaled to match the measured emission of the 50 W incandescent lamp. The good agreement between measured and calculated values indicates the quasi-ideal behavior of our white light source. The calculated peak wavelength and total irradiance are 1000 nm and 84 mW/cm2, respectively.
2.3 Photoinactivation assays
Isolated colonies of Xylella fastidiosa were suspended in 1X PBS and adjusted to a final optical density at 600 nm of ~ 1.0 by a Shimadzu UV-1800 spectrophotometer (Kyoto, Japan), corresponding to a concentration of ~ 106 CFU/mL, as previously assessed by bacterial serial dilutions on BCYE Agar and plate counting.
Photoinactivation assay in liquid medium. Effect of exposure to blue light was investigated with bacterial cells inoculated in phosphate buffer. Aliquots of 2 mL of 1X PBS with initial cell count of 7 × 105 CFU/mL (evaluated by plate counting of bacterial serial dilutions on BCYE Agar) were placed in a 6-well plate and dark incubated or illuminated with blue light for 16 min, reaching a total fluence of 100 J/cm2. At the end of the treatment, a viable count technique was performed on the samples and bacterial viability was expressed as CFU/mL by plating the samples in serial dilutions on BCYE Agar. The plates were incubated at 28 °C for at least 15–20 days. Experiments were performed at least in triplicate.
Untreated samples of X. fastidiosa suspended in 1X PBS were observed with the optical microscope AxioSkop-2 Microscope (Zeiss, Oberkochen, Germany) at the magnification of 100x. Digital images were acquired connecting the optics to an Optikam B5 camera.
Photoinactivation assays on solidified medium. Two experimental setups were performed in solidified medium. In the former one, herein called “photo-spot test” and previously optimized for microbial models by authors, samples of X. fastidiosa at ~ 106 CFU/mL were serially tenfold diluted and drops of 14 µL were inoculated on BCYE Agar. In the latter setup (“spread test”), volumes of 100 µL of cell suspension (~ 106 CFU/mL) were inoculated and spread on BCYE Agar. In both setups, inoculated cells were irradiated under 410 nm light at increasing times, 8, 16, and 32 min, to reach fluence values of 50, 100, and 200 J/cm2, respectively. For each experiment, a dark control was considered. Since irradiation caused the rise of water vapor droplets under the cover, it was necessary to leave the Petri dish uncovered to not decrease bacterial irradiation. To minimize sample contamination, no heat dissipation fan was used. This is a very crucial step since cell growth is very slow (at least 15–20 days) and even small contaminations can compromise the experiment. Furthermore, due to the black color of the growth medium, heating of the Petri dish was unavoidable. After irradiation or dark incubation, Petri dishes were sealed with top cap and parafilm and incubated at 28 °C in the dark. After 20–30 days, the bacterial growth was checked. The irradiation under wide spectrum white light was also performed for cells inoculated as previously described for “photo-spot test” for 8 and 16 min. The experiments have been performed at least three times.
2.4 Recovery of potential dormant bacteria
The following procedure was performed to search for potential dormant cells, to verify the bactericidal/bacteriostatic activity of the treatment. We evaluated whether X. fastidiosa cells incubated for 20 days at 28 °C after dark incubation or irradiation from previously described “photo-spot test” could grow on fresh medium after re-inoculum. The procedure was performed for all samples that give or not a visible bacterial growth. The bacterial biomass, visible or not at naked eye, was collected and inoculated in fresh BCYE Agar. The three samples corresponding to undiluted (ND), 10–1, and 10–2 diluted samples were recovered from cells dark incubated or irradiated at increasing fluence (50, 100, and 200 J/cm2) and incubated at 28 °C. After incubation for further 20–30 days at 28 °C, the growth was checked.
2.5 Heating curves
BCYE Agar is black due to its charcoal content. The heating effect caused by the prolonged irradiation with blue light placed 10 cm above the Petri dish was hence determined. A thermocouple was inserted just below the surface of the solid medium to record its temperature during the 32-min illumination period. Temperature was finally plotted against time to obtain the heating curve. The white light source was placed at different heights above the Petri dishes to find the distance leading to the same heating effect, and the eight of 8 cm was chosen.
2.6 Statistical analysis
Statistical analysis was assessed by one-way ANOVA in conjunction with Tukey’s post hoc test for CFU/mL values obtained with photoinactivation experiments of suspended bacteria.
3 Results and discussion
3.1 Sensitivity of Xylella fastidiosa subsp. pauca Salento-1 to light at 410 nm
Xylella fastidiosa subsp. pauca Salento-1 was isolated from an olive plant suffering from OQDS in Apulia (South Italy) and characterized by authors [24, 26]. This subspecies is known to invade olive trees through the world (California, Argentina, Brazil) [25].
With the aim of searching for anti-Xylella treatments, in this study, the potential of aBL was evaluated. LED emitting at 410 nm was preferred to 455 nm because the former showed to be more efficient in photoinactivation of Pseudomonas aeruginosa, a bacterial species belonging to γ-Proteobacteria and phylogenetically close to X. fastidiosa [23].
To observe the effect of blue light, bacterial suspensions of X. fastidiosa in phosphate buffer at a concentration ~ 105 CFU/mL were irradiated under fluence of 100 J/cm2. A significant reduction (p = 1.97 × 10–6) of ~ 2 log units was observed (Fig. 2).
Effect of irradiation on Xylella fastidiosa viability. Xylella fastidiosa subsp. pauca Salento-1 cells were suspended in PBS 1X at concentration ⁓105 CFU/mL and incubated in the dark or irradiated under light at 410 nm (100 J/cm2). The cell viability is expressed as CFU/mL (mean ± standard deviation). The experiments were carried out at least three times with independent cultures. ANOVA analysis was performed to evaluate the significant differences between irradiated and dark incubated samples (***p < 10–6). Representative images of bacterial growth deriving from plating 100 µL of the 1:100 dilution of the starting bacterial preparations are shown below histogram bars
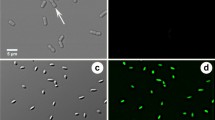
In the case of X. fastidiosa Salento-1, the culturable fraction measured as CFU on agar plates could not originate from a single cell but from a multicellular aggregate that was not completely separated by mixing with vortex and micropipette [36], as shown in Fig. 3. Since aggregation could mask the real anti-Xylella effect of blue light, further approaches were performed to verify a photoinactivation activity. With the so-called photo-spot test, undiluted and tenfold serially diluted suspensions of X. fastidiosa Salento-1 were inoculated on BCYE Agar and dark incubated or irradiated at increasing light doses. The dark incubated samples, after at least 20 days of incubation at 28 °C, showed growth spots with decreasing dimensions, thickness and colony densities, and numbers compatible with corresponding bacterial sample dilutions (Fig. 4A). An anti-Xylella effect was clear upon irradiation: no growth spot was visible for most dilutions (Fig. 4B–D). Furthermore, blue light irradiation affected the growth of undiluted samples (ND) that appeared less thick and smooth than dark controls. Indeed, under the tested conditions, the samples with the highest bacterial density were the most difficult to be inactivated. The antimicrobial effect of blue light showed a certain degree of cellular density dependence, as previously observed in P. aeruginosa PAO1 [23]. On the contrary, the highest tested fluence (~ 200 J/cm2) reached after 32 min of irradiation did not fully inhibit the growth of X. fastidiosa Salento-1 strain, as observed in PAO1 strain. It has been previously cited the hypothesis that consider the bactericidal effect of blue light as a result of the excitation of endogenous photosensitizers involved in metabolic pathways [15, 16]. Thus, the lower sensitivity of X. fastidiosa that is a slow-growing bacterium could be ascribable to a lower content of putative photosensitizers compared to that of P. aeruginosa PAO1. To the best of our knowledge, this study shows for the first time the susceptibility of X. fastidiosa to photoinactivation through light at 410 nm. Thus, endogenous compounds excitable by light at 410 nm could mediate the arising of a photooxidative stress as hypothesized for other microbial species. Indeed, porphyrin-based electron transporters could play the role of putative PSs [16]. Since it is known that X. fastidiosa is strictly aerobic and the low growth rate could be ascribable to a low rate of aerobic respiration, the content of porphyrin-based compounds could be poor [3, 27]. This could be in accordance with the lowest sensitivity observed in a fastidious growing bacterium such as X. fastidiosa compared to higher growing P. aeruginosa.
Representative images of “photo-spot test.” Xylella fastidiosa subsp. pauca Salento-1 was inoculated on BCYE Agar at decreasing bacterial concentrations. Samples of 14 µL of 106 CFU/mL bacterial suspension and decimal following dilutions were spotted and irradiated under light at 410 nm at increasing fluence rates. After 20 days of incubation at 28 °C, growth spots of dark incubated bacteria (A) were considered as control to be compared with irradiated samples (B–D). The experiments have been performed at least three times with independent cultures
A second experimental setup (“spread test”) was performed inoculating samples of 100 µL of X. fastidiosa, at ~ 106 CFU/mL, on the whole surface of BCYE Agar. As can be observed in Fig. 5, dark incubated cells after at least 20 days of incubation at 28 °C showed a confluent growth, while a drastic reduction of the bacterial growth was already induced after irradiation under the lowest fluence (50 J/cm2). Higher fluences, 100 and 200 J/cm2, respectively, showed a photoinactivation effect. Few colonies were visible close to the borders of the Petri dishes, and this could be ascribable to the geometry of irradiation from the irradiating device.
Representative images of “spread test.” A sample of 100 µL Xylella fastidiosa subsp. pauca Salento-1 at 106 CFU/mL was inoculated on BCYE agar, dark incubated or irradiated under light at 410 nm at increasing times (8, 16, and 32 min). After 20 days of incubation at 28 °C, bacterial growth of dark incubated cells (A) was compared to irradiated samples (B–D). The experiments have been performed at least three times with independent cultures
Taken as a whole, the data support that blue light impairs the viability of X. fastidiosa Salento-1. Since X. fastidiosa forms clusters and cellular aggregations before adhering and forming biofilm communities in natural environments, this technique could be applied to prevent biofilm formation. This common strategy used by plant-associated bacteria is essential for the development of mutualistic, commensal, and pathogenic host–microbe interactions [28]. Biofilm formation is likely a major virulence factor in diseases related to X. fastidiosa for establishing within the mouthparts of insect vectors [29] and enhancing the likelihood of bacterial transmission by insects [30] and for colonizing plant vessels developing into large colonies to cell aggregates similar to those visible in Fig. 3 [31,32,33].
3.2 Further investigations on the bactericidal effect of photoinactivation
The slow-growing rate of X. fastidiosa makes more difficult to evaluate the efficiency of any antimicrobial technique, including that of blue light. If bacteria grown after irradiation are not able to furtherly grow, an underestimation of the antimicrobial effect could occur. In addition, if after irradiation dormant and viable cells are present and not detectable through cultivation technique, an overestimation of antimicrobial effect could occur. This last effect could be also due to X. fastidiosa’s natural tendency to form biofilms and cell aggregates that can affect cell count measurement of the colony-forming units by both bacterial plating and optical density (OD) [34, 35].
To assess risks of bias, “recovery” experiments were performed with cells collected from the “photo-spot test.” After 20 days of incubation at 28 °C, Petri dishes from “photo-spot tests” were evaluated and both biomasses visible and not visible from spots at the lowest dilutions (undiluted “ND,” and 10–1 and 10–2 diluted samples) were collected and inoculated in fresh BCYE Agar for further 20 days at 28 °C. This procedure did not compromise the growth of the dark controls as can be appreciated in Fig. 6. Interestingly, bacterial growth was only observed with bacteria recovered after irradiation with the lowest fluence rate (50 J/cm2, 8 min of irradiation) and the highest bacterial density (ND). Thus, light at 410 nm showed a bactericidal effect at the highest fluences (≥ 100 J/cm2) irrespective of bacterial density. Indeed, these experiments confirmed that the photoinactivation of X. fastidiosa with light at 410 nm is both light-dose and bacterial density dependent.
Representative images of “recovery” experiments. Samples of Xylella fastidiosa subsp. pauca Salento-1 were photoinactivated through “photo-spot test” as depicted in Fig. 4. Unirradiated and irradiated (50, 100, and 200 200 J/cm2) cells were recovered from corresponding growth spots (ND, 10–1, and 10–2), and furtherly incubated for at least 20 days at 28 °C on fresh medium
3.3 Effect of heating on Xylella fastidiosa growth
Since the optimal temperature for X. fastidiosa is 28 °C, it was necessary to rule out any detrimental effect caused by increased temperature during treatment. Furthermore, the growth medium used for X. fastidiosa is black due to the presence of charcoal among the components and prolonged illumination could be supposed to enhance a heating effect. Thus, we therefore build the heating curves of Petri dishes containing BCYE agar and irradiated under either blue or white light. Both light sources showed a similar thermogram, with temperature rising from 28 to 40 °C within the first 15 min illumination time and then remaining constant (Fig. 7A). Thus, we evaluated the effect of incubating tenfold diluted samples at 40 °C for 8 and 16 min (Fig. 7B). A certain degree of toxicity was observed after 16 min of incubation at 40 °C for the samples at the highest dilutions. However, even if the contribution of the thermal effect cannot be ruled out, the sensitivity of X. fastidiosa to light at 410 nm is clearly shown (Figs. 4, 7 B).
A Heating curves for BCYE Agar Petri dishes irradiated under blue light (blue dots) and white light (orange dots). B Representative pictures of Xylella fastidiosa subsp. pauca Salento-1 strain untreated control and after heating at 40 °C for 8 and 16 min, respectively. All plates were inoculated by spotting 14 µL of ~ 106 CFU/mL bacterial suspension and tenfold following dilutions. C Effect of white light irradiation on X. fastidiosa for 8 min and 16 min. After 20 days of incubation at 28 °C, the growth spots were checked. The experiments have been performed at least three times with independent cultures. The comparison with effect of blue light from Fig. 4 is shown in D
To assess whether the photoinactivation of X. fastidiosa could be ascribable to a blue light-specific effect, we carried out the photo-spot test irradiating bacteria under the extended white light spectrum of a 50-W incandescent lamp. This light source was placed 8 cm above the Petri dishes, the distance at which the same thermal effect of the blue light is obtained (Fig. 7A). The bacterial growth of samples irradiated for 8 and 16 min under this light source was comparable to that of dark control, irrespective of cellular density (Fig. 7C). The observed selective activity of blue light (Fig. 7D) is in accordance with the hypothetical action mechanism proposed for aBL. The absorption of energy of visible light at a specific wavelength could induce the passage of putative endogenous photosensitizers from a ground state to an excited one. The following photochemical reactions could generate oxidizing species. In accordance with photodynamic process, type I reaction could involve direct electron or hydrogen transfer to a biomolecule, producing superoxide anion and other reactive oxygen species (ROS). While, in type II reaction, PS transfers energy to molecular oxygen, eliciting the production of singlet oxygen (1O2), characterized by extremely oxidant power and a very short lifetime [36]. The threshold of energy and/or electron transfer induced by blue light, especially at the highest fluences, seems to cause permanent damage to X. fastidiosa cells, likely at cytoplasmic and/or membrane levels.
Although X. fastidiosa is a mesophilic microorganism characterized by the optimal growth temperature 28–30 °C and the continuous incubation at 36 and 40 °C for 7-day period has been demonstrated to be lethal for the bacterial cells [37], the sunlight irradiation in Apulia Region did not prevent the diffusion of this pathogen that is or is becoming endemic. Indeed, the growth and survival of X. fastidiosa in Apulia Region both in trees and insects is probably ensured by the daily alternation of light and darkness that is a condition producing a temperature change mitigating the effects of extreme temperature during the warmer hours of the day. Moreover, it is conceivable that the anatomy and the tissue structure, as well as some physiological/adaptation strategies of the tree branches and trunks, and the insect vectors should maintain the internal temperature to conditions suitable for the life, enzyme, and cell activities, by avoiding the excessive increase of the temperature in inner compartments.
4 Conclusions
In this work, for the first time, preliminary evidences on the activity of aBL against X. fastidiosa were produced, suggesting that aBL deserves attention as a technique potentially useful to control the dissemination of X. fastidiosa. The photokilling effect is ascribable most to blue light than heating effect. Further investigations should be aimed at evaluating whether irradiation at 410 nm contributes to decrease the possible quantity of pathogens on the surface of pruning residual plant material, a phytosanitation approach for reducing potential X. fastidiosa reservoir. The combination of blue light with exogenous photosensitizers belonging to porphyrins family, eventually formulated as porphyrin-based nanomaterial and/or dyes [38], could enhance the antimicrobial effect especially against dormant forms and resistance structures, such as biofilm, reducing the risk of tolerance development and producing low environmental impact. Indeed, the visible light alone or together with natural compounds could be used for disinfection of agricultural equipment used for dissemination control.
Data availability
The data will be made available upon request.
References
Sicard, A., Zeilinger, A. R., Vanhove, M., Schartel, T. E., Beal, D. J., Daugherty, M. P., & Almeida, R. P. P. (2018). Xylella fastidiosa: Insights into an emerging plant pathogen. Annual review of Phytopathology, 56, 181–202.
Redak, R. A., Purcell, A. H., Lopes, J. R. S., Blua, M. J., Mizell, R. F., & Andersen, P. C. (2004). The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annual Review of Entomology, 49, 243–270.
Petit, G., Bleve, G., Gallo, A., Mita, G., Montanaro, G., Nuzzo, V., Zambonini, D., & Pitacco, A. (2021). Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europaea: Revisiting a tale of plant-pathogen interaction. AoB Plants, 13(4), 1–9.
Uceda-Campos, G., Feitosa-Junior, O. R., Santiago, C. R. N., Pierry, P. M., Zaini, P. A., de Santana, W. O., Martins-Junior, J., Barbosa, D., Digiampietri, L. A., Setubal, J. C., & da Silva, A. M. (2022). Comparative genomics of Xylella fastidiosa explores candidate host-specificity determinants and expands the known repertoire of mobile genetic elements and immunity systems. Microorganisms, 10(5), 914.
Saponari, M., Giampetruzzi, A., Loconsole, G., Boscia, D., & Saldarelli, P. (2019). Xylella fastidiosa in olive in Apulia: Where we stand. Phytopathology, 109(2), 175–186.
Saponari, M., Boscia, D., Altamura, G., Loconsole, G., Zicca, S., D’Attoma, G., Morelli, M., Palmisano, F., Saponari, A., Tavano, D., Savino, V. N., Dongiovanni, C., & Martelli, G. P. (2017). Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Science and Reports, 7(1), 17723.
Denancé, N., Legendre, B., Briand, M., Olivier, V., de Boisseson, C., Poliakoff, F., & Jacques, M. A. (2017). Several subspecies and sequence types are associated with the emergence of Xylella fastidiosa in natural settings in France. Plant Pathology, 66, 1054–1064.
Quetglas, B., Olmo, D., Nieto, A., Borràs, D., Adrover, F., Pedrosa, A., Montesinos, M., De Dios García, J., López, M., Juan, A., & Moralejo, E. (2022). Evaluation of control strategies for Xylella fastidiosa in the Balearic Islands. Microorganisms, 10(12), 2393.
Schaad, N. W., Postnikova, E., Lacy, G., Fatmi, M., & Chang, C. J. (2004). Xylella fastidiosa subspecies: X. fastidiosa subsp. piercei, subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov. Systematic and Applied Microbiology, 27, 290–300.
Trkulja, V., Tomić, A., Iličić, R., Nožinić, M., & Milovanović, T. P. (2022). Xylella fastidiosa in Europe: From the introduction to the current status. Plant Pathology Journal, 38, 551–571.
da Silva, D. F., Amaral, J. C., Carlos, R. M., Ferreira, A. G., Forim, M. R., Fernandes, J. B., da Silva, M. F. G. F., Filho, H. D. C., & de Souza, A. A. (2021). Octahedral ruthenium and magnesium naringenin 5-alkoxide complexes: NMR analysis of diastereoisomers and in-vivo antibacterial activity against Xylella fastidiosa. Talanta, 225, 122040.
Baró, A., Badosa, E., Montesinos, L., Feliu, L., Planas, M., Montesinos, E., & Bonaterra, A. (2020). Screening and identification of BP100 peptide conjugates active against Xylella fastidiosa using a viability-qPCR method. BMC Microbiology, 20, 1–14.
Baró, A., Mora, I., Montesinos, L., & Montesinos, E. (2020). Differential susceptibility of Xylella fastidiosa strains to synthetic bactericidal peptides. Phytopathology, 110, 1018–1026.
Baldassarre, F., Tatulli, G., Vergaro, V., Mariano, S., Scala, V., Nobile, C., Pucci, N., Dini, L., Loreti, S., & Ciccarella, G. (2020). Sonication-assisted production of fosetyl-Al nanocrystals: Investigation of human toxicity and in vitro antibacterial efficacy against Xylella fastidiosa. Nanomaterials, 10, 1–20.
Wang, Y., Wang, Y., Wang, Y., Murray, C. K., Hamblin, M. R., Hooper, D. C., & Dai, T. (2017). Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resistance Update, 33–35, 1–22.
Lubart, R., Lipovski, A., Nitzan, Y., & Friedmann, H. (2011). A possible mechanism for the bactericidal effect of visible light. Laser Theraphy, 20, 17–22.
Wang, Y., Wu, X., Chen, J., Amin, R., Lu, M., Bhayana, B., Zhao, J., Murray, C. K., Hamblin, M. R., Hooper, D. C., & Dai, T. (2016). Antimicrobial blue light inactivation of Gram-Negative pathogens in biofilms: In vitro and in vivo studies. Journal of Infectious Diseases, 213, 1380–1387.
Dai, T., & Hamblin, M. R. (2017). Visible blue light is capable of inactivating Candida albicans and other fungal species. Photomedicine and Laser Surgery, 35, 345–346.
Amin, R. M., Bhayana, B., Hamblin, M. R., & Dai, T. (2016). Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers in Surgery and Medicine, 48, 562–568.
Zhang, Y., Zhu, Y., Gupta, A., Huang, Y., Murray, C. K., Vrahas, M. S., Sherwood, M. E., Baer, D. G., Hamblin, M. R., & Dai, T. (2014). Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. Journal of Infectious Diseases, 209, 1963–1971.
Zhang, Y., Zhu, Y., Chen, J., Wang, Y., Sherwood, M. E., Murray, C. K., Vrahas, M. S., Hooper, D. C., Hamblin, M. R., Zhang, Y., Zhu, Y., Chen, J., Wang, Y., Margaret, E., Murray, C. K., Vrahas, M. S., Hooper, D. C., & Hamblin, M. R. (2016). Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence, 7, 536–545.
Rapacka-Zdonczyk, A., Wozniak, A., Pieranski, M., Woziwodzka, A., Bielawski, K. P., & Grinholc, M. (2019). Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sub-lethal treatment. Science and Reports, 9, 1–18.
Martegani, E., Bolognese, F., Trivellin, N., & Orlandi, V. T. (2020). Effect of blue light at 410 and 455 nm on Pseudomonas aeruginosa biofilm. Journal of Photochemistry and Photobiology, B: Biology, 204, 111790.
Bleve, G., Gallo, A., Altomare, C., Vurro, M., Maiorano, G., Cardinali, A., D’Antuono, I., Marchi, G., & Mita, G. (2018). In vitro activity of antimicrobial compounds against Xylella fastidiosa, the causal agent of the olive quick decline syndrome in Apulia (Italy). FEMS Microbiology Letters, 365, 281.
Ramazzotti, M., Cimaglia, F., Gallo, A., Ranaldi, F., Surico, G., Mita, G., Bleve, G., & Marchi, G. (2018). Insights on a founder effect: The case of Xylella fastidiosa in the Salento area of Apulia, Italy. Phytopathologia Mediterranea, 57, 8–25.
Bleve, G., Marchi, G., Ranaldi, F., Gallo, A., Cimaglia, F., Logrieco, F., Mita, G., Ristori, J., & Surico, G. (2016). Molecular characteristics of a strain (Salento-1) of Xylella fastidiosa isolated in Apulia (Italy) from an olive plant with the quick decline syndrome. Phytopathologia Mediterranea, 54, 75–82.
Baldi, P., & La Porta, N. (2017). Xylella fastidiosa: Host range and advance in molecular identification techniques. Frontiers in Plant Science, 8, 944.
Danhorn, T., & Fuqua, C. (2007). Biofilm formation by plant-associated bacteria. Annual Review of Microbiology, 61, 401–422.
Almeida, R. P. P., & Purcell, A. H. (2006). Patterns of Xylella fastidiosa colonization on the precibarium of sharpshooter vectors relative to transmission to plants. Annals Entomological Society of America, 99(5), 884–890.
Alves, E., Leite, B., Marucci, R. C., Pascholati, S. F., Lopes, J. R. S., & Andersen, P. C. (2008). Retention sites for Xylella fastidiosa in four sharpshooter vectors (Hemiptera: Cicadellidae) analyzed by scanning electron microscopy. Current Microbiology, 56, 531–538.
Killiny, N., Hernandez Martinez, R., Korsi Dumenyo, C., Cooksey, D. A., & Almeida, R. P. P. (2013). The exopolysaccharide of Xylella fastidiosa is essential for biofilm formation, plant virulence, and vector transmission. Molecular Plant-Microbe Interactions, 26, 1044–1053.
Newman, K. L., Almeida, R. P. P., Purcell, A. H., & Lindow, S. E. (2003). Use of a Green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Applied and Environment Microbiology, 69, 7319–7327.
Alves, E. E., Marucci, C. R., Lopes, J. R. S., & Leite, B. (2004). Leaf symptoms on plum, coffee and citrus and the relationship with the extent of xylem vessels colonized by Xylella fastidiosa. Journal of Phytopathology, 152, 291–297.
Campanharo, J. C., Lemos, M. V. F., & De Macedo Lemos, E. G. (2003). Growth optimization procedures for the phytopathogen Xylella fastidiosa. Current Microbiology, 46, 99–102.
Navarrete, F., & De La Fuente, L. (2014). Response of Xylella fastidiosa to zinc: Decreased culturability, increased exopolysaccharide production, and formation of resilient biofilms under flow conditions. Applied and Environment Microbiology, 80, 1097–1107.
Hadi, J., Wu, S., Brightwell, G., & Hadi, J. (2020). Antimicrobial blue light versus pathogenic bacteria: Mechanism, application in the food industry, hurdle technologies and potential resistance. Foods, 9(12), 1895.
Román-Écija, M., Landa, B. B., Testi, L., & Navas-Cortes, J. A. (2021). Extreme temperature differentially affects growth and survival of Xylella fastidiosa strains. Proceedings of the 3rd European Conference on Xylella fastidiosa and XF-ACTORS Final Meeting, 26-30 April 2021, Online Event https://doi.org/10.5281/zenodo.4882653
Ferreira, J. R. M., Sierra-Garcia, I. N., Guieu, S., Silva, A. M. S., da Silva, R. N., & Cunha, Â. (2021). Photodynamic control of citrus crop diseases. World Journal of Microbiology and Biotechnology, 3(12), 199.
Funding
Open access funding provided by Università degli Studi dell'Insubria within the CRUI-CARE Agreement. The present research was partially supported by INTEGROLIV project “Integrated Eco-friendly Approach for the Containment of Xylella fastidiosa and for the Regeneration of Olive Growing and the Environment (Project Code H33C22000860001)––Minister of Agriculture, Food Sovereignty and Forests Italy (MASAF), D.M. n. 664829 del 29/12/2022––Research Topic 2: Investigations and tests to identify methods of control of Xylella fastidiosa. It has received financial support from “PON Ricerca e Innovazione 2014–2020,” Asse IV “Istruzione e ricerca per il recupero” Azione IV.4 “Dottorati e contratti di ricerca a carattere industriale su tematiche dell’innovazione,” A.Y. 2022–23, XXXVII Cycle, for the PhD project grant of Annamaria Tarantini.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bleve, G., Trivellin, N., Chirizzi, D. et al. Sensitivity of Xylella fastidiosa subsp. pauca Salento-1 to light at 410 nm. Photochem Photobiol Sci 23, 793–801 (2024). https://doi.org/10.1007/s43630-024-00556-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-024-00556-z